Exploring the mechanism of action of Curcuma zedoaria and Biejia medicine in combating liver fibrosis based on network pharmacology, molecular docking, and experimental verification
Liver fibrosis refers to the chronic inflammation and necrosis of liver cells caused by the continuous action of various pathogenic factors. Some non parenchymal cells in the liver are activated, resulting in diffuse extracellular matrix proliferation, fibrous proliferation, and imbalanced fiber decomposition in the liver, leading to fibrous connective tissue proliferation in the liver and initiating the process of liver fibrosis. In the later stage, liver lobule remodeling and pseudolobule formation occur, and the liver texture becomes hard, leading to liver dysfunction, which is called cirrhosis; Liver fibrosis is not an independent disease, but a common pathological process in many chronic liver diseases. A survey shows that over 50 million adults worldwide suffer from chronic liver disease; In China, about 300 million people are affected by liver disease, accounting for 11% of global deaths from cirrhosis. Therefore, finding drugs to prevent and treat liver fibrosis is of great significance for patients with liver diseases such as liver fibrosis. According to research, traditional Chinese medicine and compound preparations (such as Compound Biejia Ruangan Tablets/Capsules, Fuzheng Huayu Tablets/Capsules, Dahuang Zhechong Pills, etc.) have good therapeutic effects in preventing and treating liver fibrosis, and have the advantages of multiple components and targets.
Liver fibrosis belongs to the categories of “pain relief”, “liver obstruction”, “liver accumulation”, and “accumulation” in traditional Chinese medicine. Curcumae Rhizoma (CR) and Trionycis Carapax (TC) are commonly used drugs in the clinical treatment of liver fibrosis in traditional Chinese medicine. TC enters the liver, softens the hard and disperses nodules, removes blood stasis and eliminates symptoms. CR promotes blood circulation and removes blood stasis, softens the hard and promotes stagnation, and eliminates symptoms and nodules. The two are used in combination to increase the effect of promoting blood circulation, removing blood stasis, and softens the hard and disperses nodules. Modern pharmacological studies have shown that CR can exert its anti liver fibrosis effects through multiple mechanisms of action. In addition, various traditional Chinese medicine compound preparations composed of TC can improve patients’ liver function and alleviate fibrosis process. Clinical and experimental studies suggest that both CR and TC have clear anti liver fibrosis effects, but the specific mechanism of their synergistic anti liver fibrosis effect when combined as a drug has not been elucidated. This study adopts various research methods such as network pharmacology, molecular docking technology, and experimental verification to explore the role and possible mechanisms of traditional drugs in the anti liver fibrosis of CRTC from the aspects of components, targets, biological functions, signaling pathways, etc., hoping to provide a certain basis and reference for modern research on CRTC’s anti liver fibrosis.
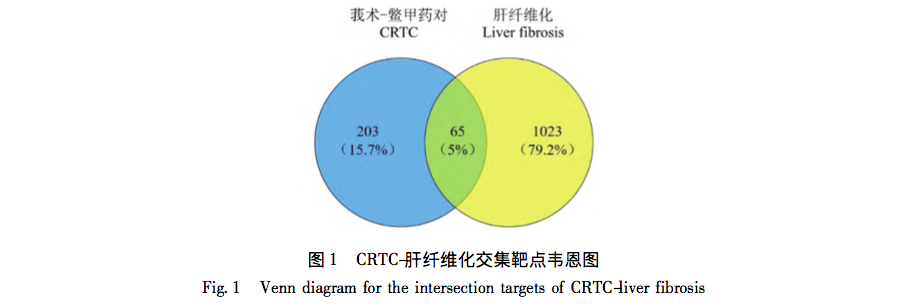
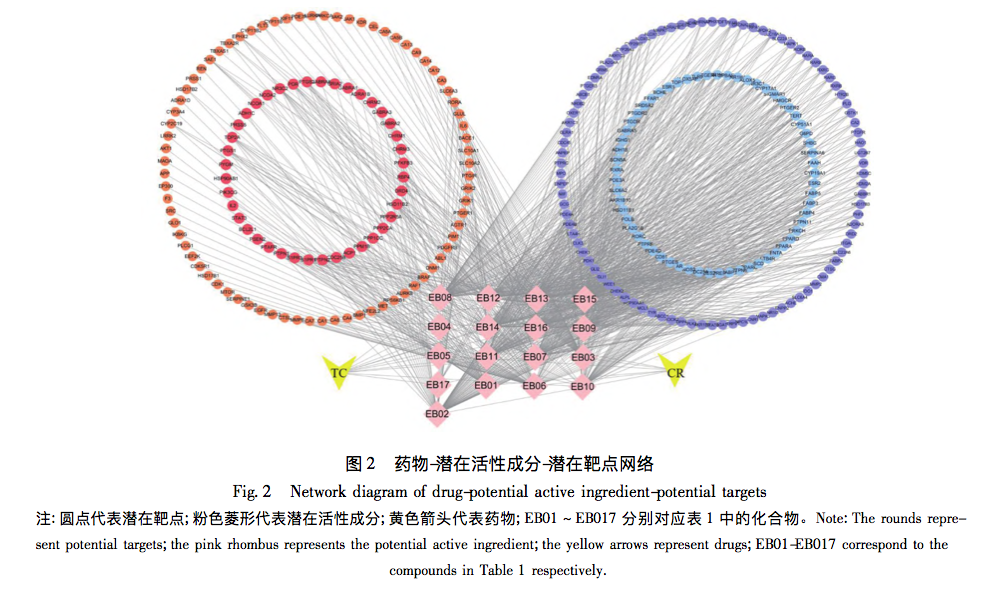





 The study of the modern characteristics and mechanisms of traditional Chinese medicine has always been a key focus of modern research in traditional Chinese medicine. Biejia and Curcuma zedoaria are commonly used drugs in the clinical treatment of liver fibrosis in traditional Chinese medicine. In clinical practice, the two are often used interchangeably, exerting the effects of promoting blood circulation, removing blood stasis, softening and dispersing nodules, and having a clear anti liver fibrosis effect. However, the specific mechanism of synergistic anti liver fibrosis effects of these two drugs has not been clear for a long time.
The study of the modern characteristics and mechanisms of traditional Chinese medicine has always been a key focus of modern research in traditional Chinese medicine. Biejia and Curcuma zedoaria are commonly used drugs in the clinical treatment of liver fibrosis in traditional Chinese medicine. In clinical practice, the two are often used interchangeably, exerting the effects of promoting blood circulation, removing blood stasis, softening and dispersing nodules, and having a clear anti liver fibrosis effect. However, the specific mechanism of synergistic anti liver fibrosis effects of these two drugs has not been clear for a long time.
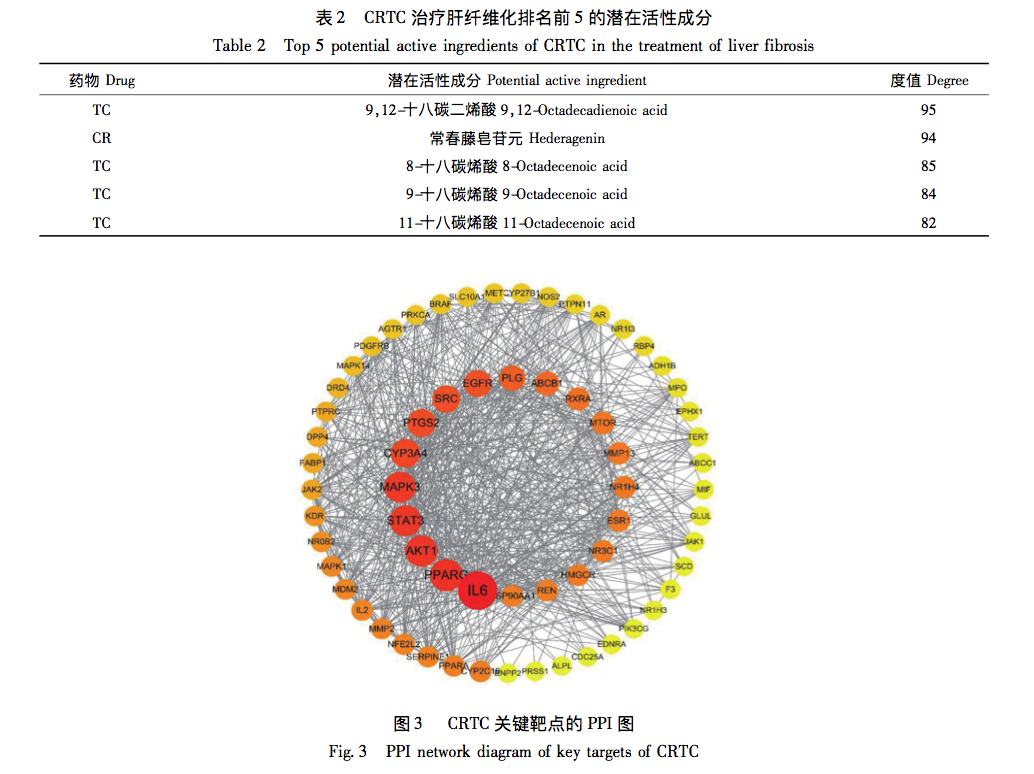
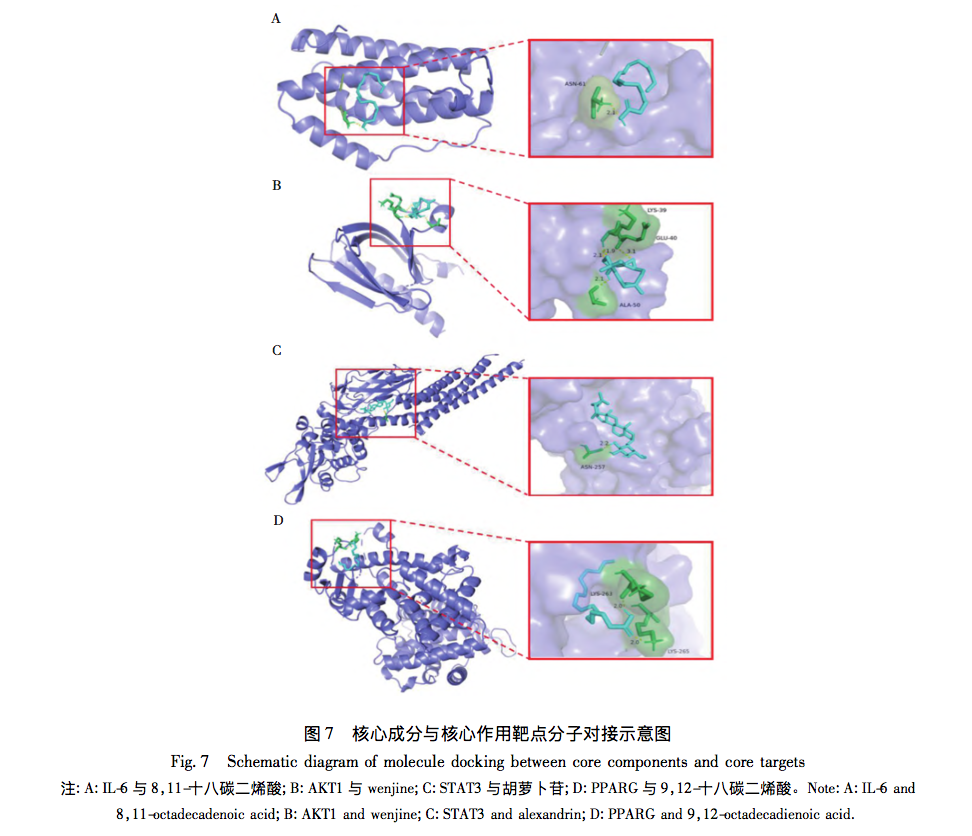
This article uses modern network pharmacology to first screen the intersection targets of CRTC and liver fibrosis, and then create a PPI network to screen core targets such as IL-6, AKT1, STAT3, PPARG, etc. Among them, IL-6 is the key target with the highest degree ranking. IL-6 is a member of the pro-inflammatory cytokine family and can affect various immune and physiological processes, such as acute phase protein production (such as C-reactive protein, ferritin, etc.), inflammation, antigen-specific immune response, hematopoiesis, cell apoptosis, differentiation, and cell metabolism. In addition, studies have found that the activation of STAT3 signaling in liver cells usually improves liver inflammation and regulates the process of liver fibrosis by preventing liver cell damage and inhibiting STAT1 signaling; STAT3 can also regulate the transition from fibroblasts to myofibroblasts and the transcription of matrix metalloproteinases and their homologous inhibitory proteins, thereby improving liver fibrosis at the extracellular matrix degradation level. Analysis shows that IL-6 and STAT3 are of great significance for CRTC in combating liver fibrosis.
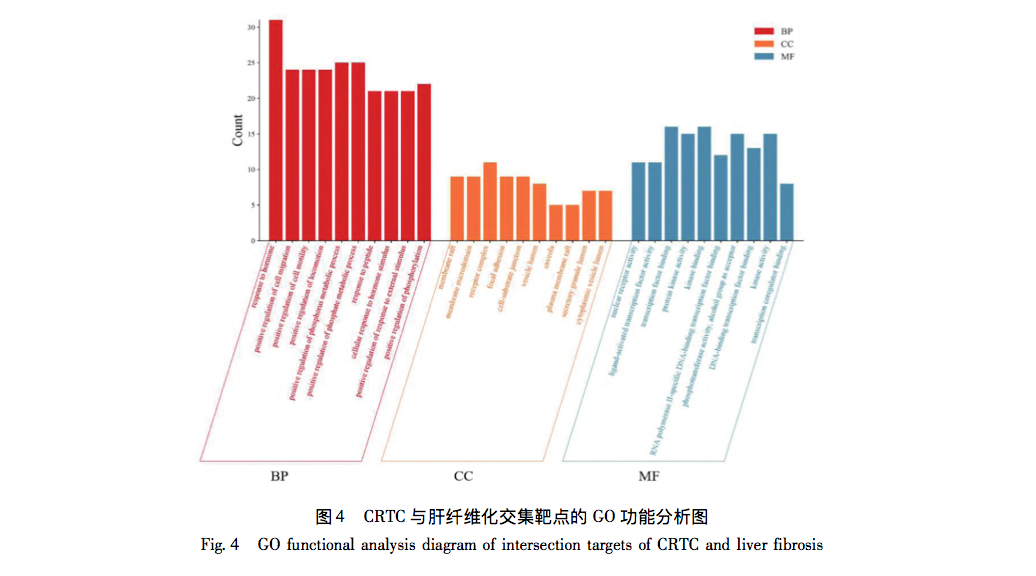
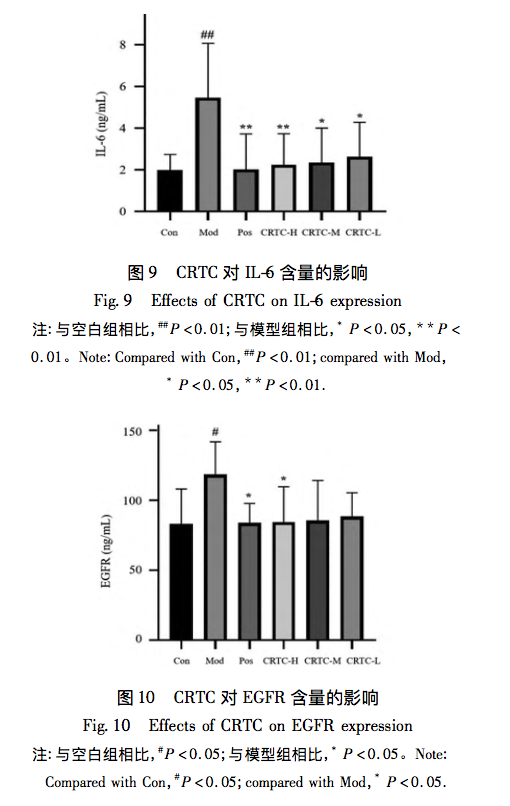
Further analysis of the network model using topological analysis revealed that the pharmacological effects of CRTC’s multi-component multi-target form a complex network. Five key functional components were identified, namely 9,12-octadecadienoic acid, hederagenin, 8-octadecadienoic acid, 9-octadecadienoic acid, and 11 octadecadienoic acid. The main biological processes involved in its anti liver fibrosis effect include hormone response, positive regulation of phosphate metabolism, and positive regulation of phosphate metabolism. The cellular components involve receptor complexes, membrane rafts, membrane microregions, etc. The molecular functions mainly involve transcription factor binding, kinase binding, kinase activity, etc; The core signaling pathways include EGFR tyrosine kinase inhibitor resistance, PI3K Akt signaling pathway, etc. We found that in KEGG pathway enrichment analysis, the P-value of EGFR tyrosine kinase inhibitor resistance was relatively small, and there were more genes enriched in this pathway. The core targets IL-6, AKT1, STAT3, etc. were all enriched in the EGFR tyrosine kinase inhibitor resistance pathway. The EGFR tyrosine kinase inhibitor resistance pathway is of great significance for CRTC’s anti liver fibrosis effect, and the signal network formed by its interaction with JAK-STAT, PI3K Akt and other pathways cannot be ignored in its mechanism of action. Multiple studies have shown that the IL-6/EGFR/STAT signaling axis is involved in various important biological processes such as cell growth, proliferation, differentiation, apoptosis, and immune regulation. IL-6, as an important inflammatory mediator in the body’s immune response, can promote the activation of JAK/STAT signaling. When it binds to receptors, it can promote the activation of JAK1 and the phosphorylation and transcription of STAT3. Most studies have shown that STAT3 is frequently activated in almost all fibrotic systems; In the liver, STAT3 signaling is a key anti-inflammatory signal that controls liver inflammation, and activation of it can play anti-inflammatory and immune regulatory roles in the process of liver fibrosis. In addition, the use of STAT3 inhibitors in preclinical animal models has shown good anti fibrotic activity, indicating that STAT3 is the true target for treating fibrotic diseases in patients. EGFR is known to be a key regulatory factor for liver cell proliferation during the initial stage of liver regeneration; Among all tissues, EGFR is expressed highest in adult liver cells, indicating its important role in maintaining liver function. Firstly, EGF is a known soluble mediator involved in HSC activation, and EGFR can mediate HSC activation and promote a pro fibrotic phenotype. Its exact mechanism may involve the production of reactive oxygen species in liver tissue. Secondly, Fuchs et al. found in their study that EGFR inhibitors can inhibit the expression of several known pro fibrotic genes in multiple liver fibrosis models, which is the first evidence that inhibiting EGFR can reverse liver fibrosis. Finally, EGFR can bind to its specific ligands to form dimers, triggering phosphorylation of various effector proteins and activating multiple classical downstream signaling pathways, such as the signal transducer and transcriptional activator (STAT) pathways; EGFR, as a key participant in all stages of liver response to injury, can induce classical STAT3 Tyr705 phosphorylation, thereby controlling cytokine proliferation and differentiation, and inhibiting fibrosis formation. Therefore, predicting the core targets and pathways through network pharmacology, and further verifying the regulatory effect of the predicted IL-6/EGFR/STAT3 signaling axis on liver fibrosis through experiments, will help us understand the mechanism of CRTC’s anti liver fibrosis effect.
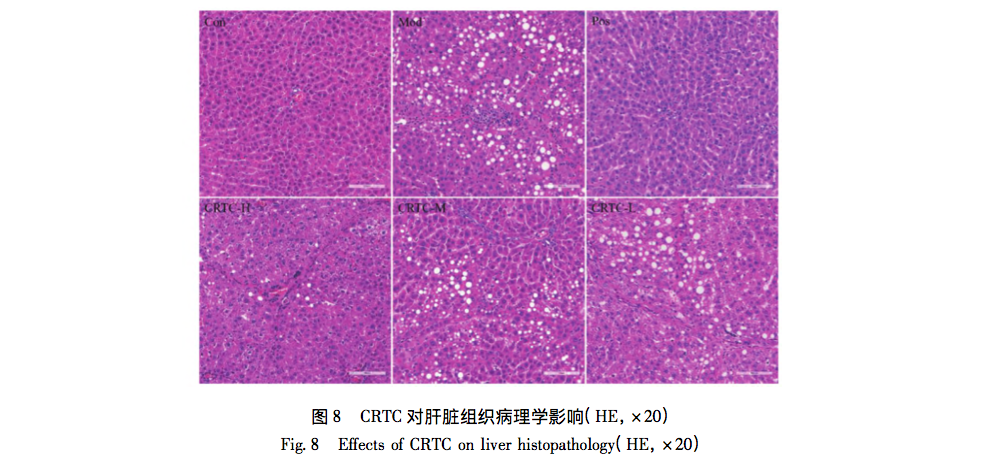
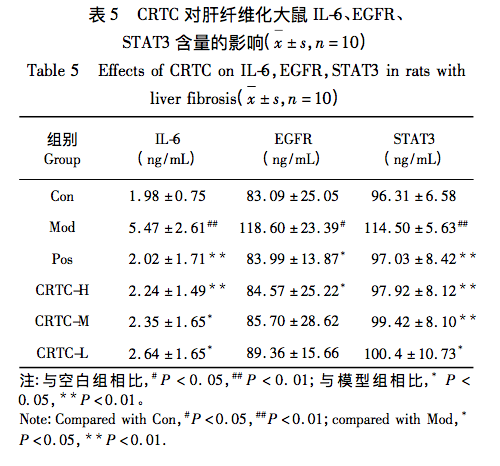
In animal experiments, HE staining results showed improvement in pathological morphology such as inflammation and infiltration after administration, indicating that CRTC can alleviate liver fibrosis caused by pig serum. Further ELISA results showed that the levels of IL-6, EGFR, and STAT3 in the serum of the model group rats were significantly increased, and all were significantly downregulated after administration, showing a dose-dependent relationship. This study suggests that the mechanism of action of CRTC in treating liver fibrosis may be related to the IL-6/EGFR/STAT3 signaling axis, providing a certain basis for revealing the modern mechanism of traditional CRTC treatment of liver fibrosis, and also providing useful references for the molecular mechanism research of network pharmacology and molecular docking technology for traditional drugs.
