On December 31, 2023, the State Administration of Market Supervision and Administration, the National Health Commission and the State Administration of Traditional Chinese Medicine issued the Announcement on the Release of the Catalogue of Ginseng and Three Other Health Food Raw Materials, which will be implemented from May 1, 2024 onwards. The update of the catalog of health food raw materials enriches the variety of products on record, reduces the cost of research and development, shortens the time to market, and is more conducive to promoting the efficient development of the health food industry. What substances are included in the catalog of health food ingredients and what are the compounding requirements of these substances are summarized by FoodPartner.com for your reference.
One
Catalog of Health Food Ingredients
Health food raw material catalog is a dynamic catalog, the State Administration of Market Supervision, in conjunction with the National Health Commission, the State Administration of Traditional Chinese Medicine, to develop, adjust and publish health food raw material catalog. Currently, the catalog of health food ingredients can be divided into two categories in accordance with the division of health care functions, one is a nutrient supplement raw materials, and the other is a non-nutrient supplement raw materials.
1, nutrient supplements raw materials catalog: contains calcium, magnesium and other 24 nutrients, corresponding to a variety of sources of compounds, the scope of application, daily dosage, efficacy, etc. need to meet the “Health Food Raw Material Catalog Nutrient Supplements (2023 Edition)” requirements.
2, Non-nutrient Supplements Raw Material Catalog: Since 2020, there are three batches of Non-nutrient Supplements Raw Material Catalog released, with a total of 10 substances. The raw material requirements are detailed in the following table.
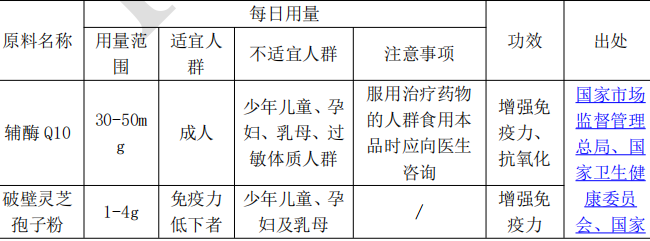

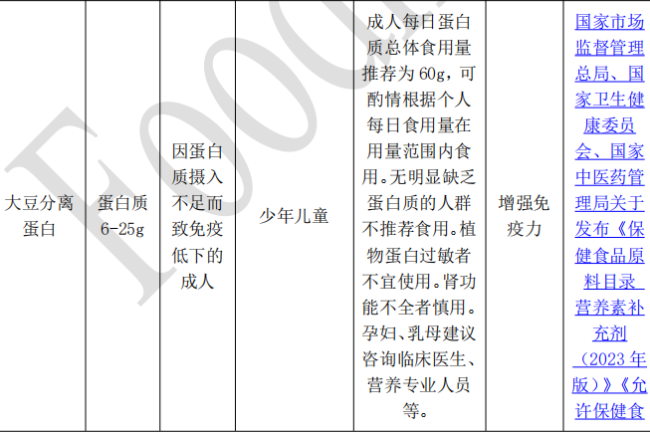

II. Requirements for compounding of substances in the catalog of health food ingredients
Health food formulations should be scientific in nature, and product ingredients need to have instructions for rational use. Regarding the compounding requirements of the 10 substances in the catalog of health food ingredients, please refer to the following information for details:
1. 4 raw materials, namely Coenzyme Q10, Wall-broken Ganoderma Lucidum Spore Powder, Spirulina and Fish Oil, cannot be used in combination with any other raw materials;
2、Melatonin can choose whether to be used in combination with vitamin B6;
3, soybean isolate protein and whey protein in the product filing, can be used alone as raw materials, you can also use the two compound. Need to be paired with nutrients, the filer should provide the same formula with the approved product types of raw materials, the same health care function of the information;
4, ginseng, western ginseng and ganoderma lucidum, when the product is filed, only a single ingredient can be used, and cannot be compounded with other raw materials.
Summary
The above is the substances in the catalog of health food ingredients summarized by FoodPartner.com in combination with relevant regulations. FoodPartner.com will continue to update and analyze the knowledge points related to health food and other special food products, so please look forward to it.
