Research on the Quality Evaluation of Verbena and the Transfer Law of Standard Decoction Dosage Based on UPLC Feature Map and One Test Multiple Evaluation Method
Verbena officinalis L., a dry aboveground part of the Verbena officinalis L. plant in the Verbenaceae family, was first recorded in the “Catalogue of Famous Physicians” and classified as a lower grade. The chemical components of verbena are mainly iridoid glycosides, flavonoids, sterols, triterpenes, polyphenols, volatile oils, tannins, beta carotene, caffeic acid, sugars, etc. The main active ingredients are iridoid glycosides. It has a cool nature, a bitter taste, and belongs to the liver and spleen meridians. It has the effects of promoting blood circulation, dispersing blood stasis, detoxifying, promoting diuresis, reducing jaundice, and intercepting malaria. Clinically, it is used for the accumulation of symptoms such as dysmenorrhea, amenorrhea, pharyngitis, abscess, edema, jaundice, and malaria. The resources of verbena are abundant, among which iridoid glycosides have various effects such as antioxidant, hepatoprotective, neuroprotective, heart and brain ischemia protective, anti-inflammatory, etc. Chinese herbal decoctions (standard decoctions) are the most commonly used traditional Chinese medicine compound preparations in clinical practice, and also the longest and most widely used preparations in the history of Chinese medicine. Whether it is traditional Chinese medicine decoction pieces, broken wall decoction pieces, traditional Chinese medicine formula granules and other new Chinese medicine decoction pieces, they are all taken by decoction or water flushing. Therefore, it is of great significance to use standard decoction research as the preferred control for the study of other Chinese medicine preparations. This study elucidates the quantitative transfer law from decoction pieces to standard decoctions, laying the foundation for the research and quality evaluation of traditional Chinese medicine formulations of verbena.
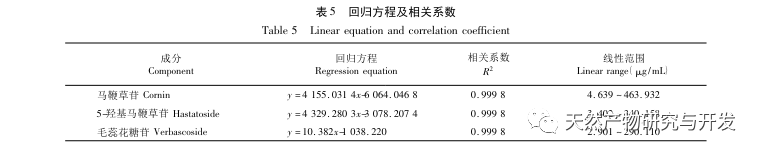
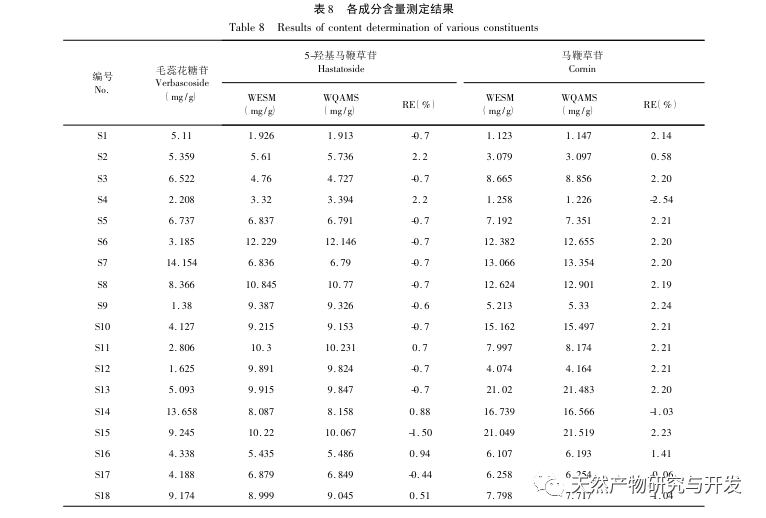
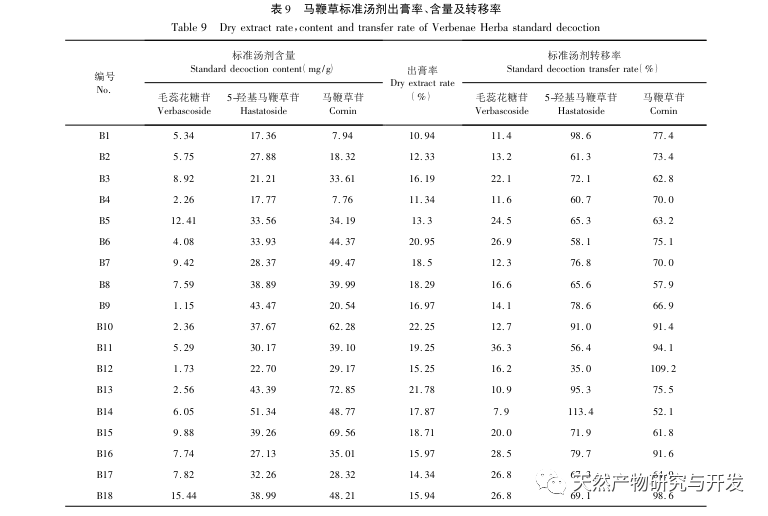
This study focuses on the preparation of verbascoside, 5-hydroxyverbascoside, and verbascoside in verbascoside slices, and establishes its UPLC characteristic map. The map information is analyzed using chemical mode analysis, and the content of verbascoside, 5-hydroxyverbascoside, and verbascoside in verbascoside is simultaneously determined by ultra-high performance liquid chromatography combined with one test multiple evaluation method. The results are compared with those obtained by external standard method. And measure the standard decoction yield, verbascoside, 5-hydroxyverbascoside, and verbascoside content, calculate the transfer rate, and based on this, conduct a study on the transfer of verbascoside decoction pieces to the standard decoction to demonstrate the rationality of various quality indicators.
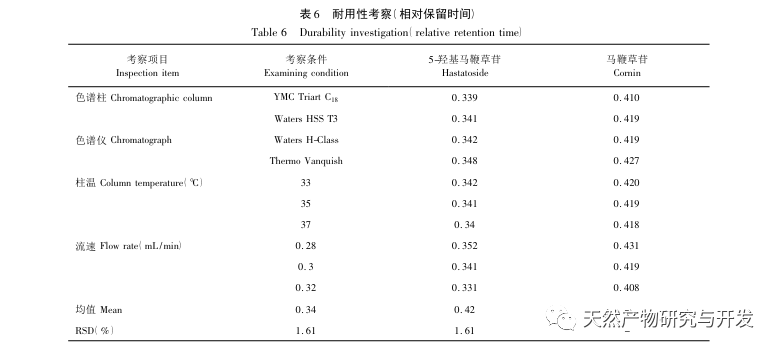
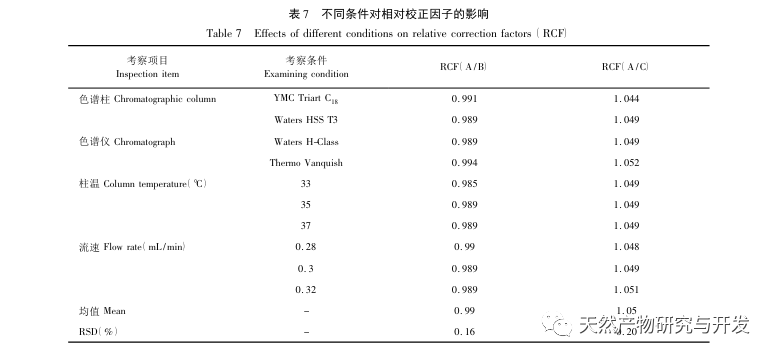
The isocratic elution of the characteristic spectrum of the verbena sample cannot achieve baseline separation, so gradient elution was used. In the preliminary experiments, this study compared mobile phases such as methanol water, acetonitrile water, acetonitrile-0.1% phosphoric acid solution, and acetonitrile-0.05% phosphoric acid solution. Finally, acetonitrile-0.05% phosphoric acid solution was selected as the mobile phase. Under the above chromatographic conditions, the chromatographic peak separation, theoretical plate number, and peak shape parameters were all good. This study used a single factor experimental system to investigate the effects of extraction solvent, extraction method and time, and solid-liquid ratio on extraction efficiency. The results showed that the extraction efficiency was highest when the extraction solvent was 80% methanol, the solid-liquid ratio was 1:50 (g/mL), and reflux extraction was carried out for 120 minutes. Therefore, the above method was used as the extraction method for verbena slices.
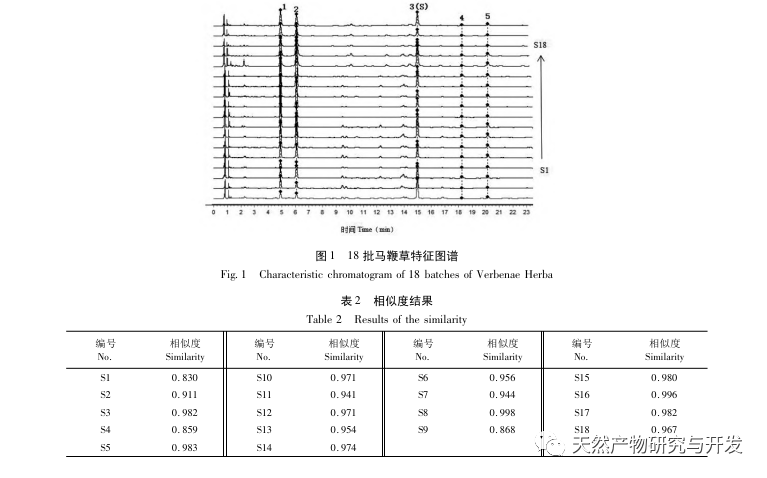
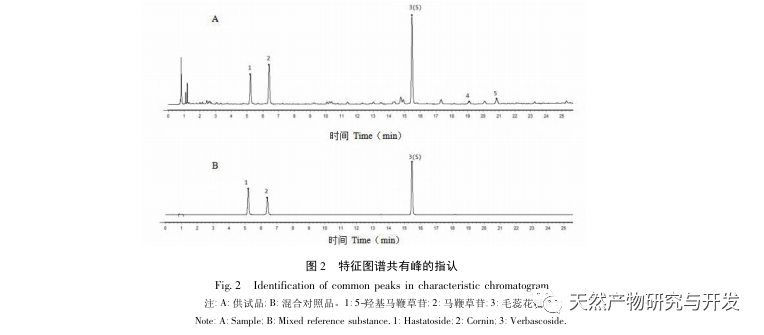
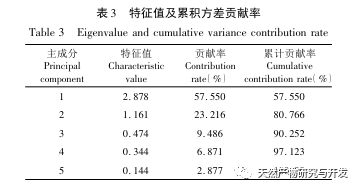
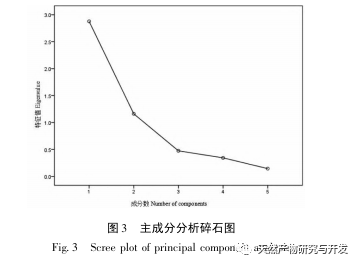
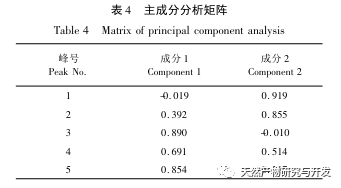
The overall quality control of multi index components in traditional Chinese medicine has become an inevitable trend in the development of traditional Chinese medicine quality control. The characteristic/fingerprint spectrum is an effective quality evaluation method, and combining it with the one test multi evaluation analysis method can achieve the integrity and accuracy of quality evaluation. This study established a UPLC characteristic map of verbascoside decoction pieces, identified 5 common peaks and identified 3 components. The analysis time was significantly shortened compared to HPLC, and the chromatographic conditions could be used for the determination of 5-hydroxyverbascoside, verbascoside, and verbascoside content. The principal component analysis was conducted on the peak area of characteristic peaks, and the comprehensive evaluation model of principal components further improved the quality evaluation and analysis method of verbena.
The content determination index of verbena in the 2020 Chinese Pharmacopoeia is the total amount of oleanolic acid and ursolic acid, both of which are triterpenoids with low polarity and low water solubility. Preliminary experimental research results show that the total content of oleanolic acid and ursolic acid in the standard decoction of verbena is less than 2.0mg/g, and the transfer rate is less than 6%. Therefore, based on the feature map, a multi evaluation method was established for the determination of the content of 5-hydroxy verbascoside, verbascoside, and verbascoside. The polar components of verbena are mainly cyclohexene ether terpenes, among which verbascoside and 5-hydroxyverbascoside are the main components with the highest content, and the standard decoction has a higher transfer rate. The transfer rate of some batches exceeds 100%, while the total transfer range of 5-hydroxyverbascoside and verbascoside is between 56.6% and 91.2%. The possible reason is that similar components undergo structural transformation during heating, boiling, and other processes. There are differences in the content and transfer rate of verbascoside, 5-hydroxyverbascoside, and verbascoside among the 18 batches of standard decoction of verbascoside, which may be related to the content and texture of the decoction pieces. Some decoction pieces have lighter colors, longer storage times, and more debris during processing, which may be the reason for the large fluctuations in their content and transfer rate. The three selected ingredients have a high content in verbena, and have good pharmacological activities in antioxidant, hepatoprotective, neuroprotective, heart and brain ischemia protective, anti-inflammatory and other aspects. They are one of the main material bases for verbena to exert its pharmacological effects.
The results of the one test multiple evaluation method showed no significant difference compared to the external standard method, and can be used for quantitative analysis of multiple components in verbena. The UPLC feature map combined with multi index component one measurement multiple evaluation method can provide reference for the research and quality evaluation of the production process of verbena and verbena Chinese herbal preparations.
