Study on HPLC characteristic spectra and chemical pattern recognition of triterpenoids in Poria cocos peel and Poria cocos
Poria cocos, also known as Futu, Fuling, Songfu, etc., is a dried fungus of the Poria cocos (Schw.) Wolf family. It has a sweet and light taste, a mild nature, and can be found in the heart, lungs, spleen, and kidney meridians. It has the effects of promoting water infiltration, strengthening the spleen, and calming the heart. Fuling is included in the 2020 edition of the Pharmacopoeia of the People’s Republic of China (referred to as the “Chinese Pharmacopoeia”), and is one of the Four Treasures and Eight Treasures of Traditional Chinese Medicine, known as the “Ten Formulas and Nine Ling”. The main pharmacological substances of Poria cocos are Poria cocos polysaccharides and Poria cocos triterpenoids. Modern pharmacological studies have shown that Poria cocos triterpenoids have diuretic, anti-tumor, immune regulatory, anti-inflammatory, anti-aging, hypoglycemic, and lipid-lowering effects, and are currently important indicators used to characterize the quality and efficacy of Poria cocos. At present, the main varieties of Poria cocos circulating in the market are cultivated varieties, and different origins, processing methods, and specifications of Poria cocos slices may affect the quality of Poria cocos medicinal materials and slices. The 2020 edition of the Chinese Pharmacopoeia only uses thin-layer chromatography to identify and control the quality of Poria cocos and Poria cocos bark medicinal materials, which is difficult to fully reflect their quality. Fingerprint analysis is one of the widely recognized and widely used methods for quality control of traditional Chinese medicine in the field of modern Chinese medicine. It has the advantages of high integrity and strong characteristics, and is in line with the comprehensive effects of multiple components in traditional Chinese medicine. It has the potential for further identification, recognition, and classification. Chemical pattern recognition can analyze the multidimensional information of fingerprint spectra, accurately reflect the quality differences of medicinal materials, and achieve the goal of overall description and reasonable evaluation of medicinal material quality. This study aims to establish HPLC fingerprint spectra of Poria cocos (derived from the epidermis of Poria cocos) and Poria cocos (derived from the peeled part of Poria cocos), and analyze the effects of each common peak on the fingerprint spectra using pattern recognition methods. The main peaks and characteristic peaks will be identified to evaluate the differences in various chemical substances between Poria cocos and Poria cocos as a whole, providing a basis for quality control of Poria cocos medicinal materials.
This study used methanol as the extraction solvent and ultrasound treatment for 30 minutes to investigate different mobile phases (acetonitrile water, acetonitrile-0.1% formic acid water, acetonitrile-0.1% phosphoric acid water, acetonitrile-0.2% phosphoric acid water, acetonitrile-0.3% phosphoric acid water), different chromatographic columns [Agilent 5 TC-C18 (2) (250mm × 4.6mm, 5 μ m), Welch Ultimate XB-C18 (250mm × 4.6mm, 5 μ m), Agilent Eclipse Plus C18 (250mm × 4.6mm, 5 μ m)], different volume flow rates (1.0, 1.1, 1.2mL/min), different column temperatures (22, 25, 28 ℃), and different collection wavelengths (203, 210, 242)]. The influence of nm on the fingerprint spectrum was ultimately determined, and the chromatographic conditions were determined to be acetonitrile 0.3% phosphoric acid water as the mobile phase, Agilent 5 TC-C18 (2) (250mm × 4.6mm, 5 μ m) chromatographic column, with a volume flow rate of 1.0mL/min, column temperature of 25 ° C, and variable wavelength acquisition. Under these conditions, the baseline is stable, the chromatographic peak shape is good, and the separation effect is good.
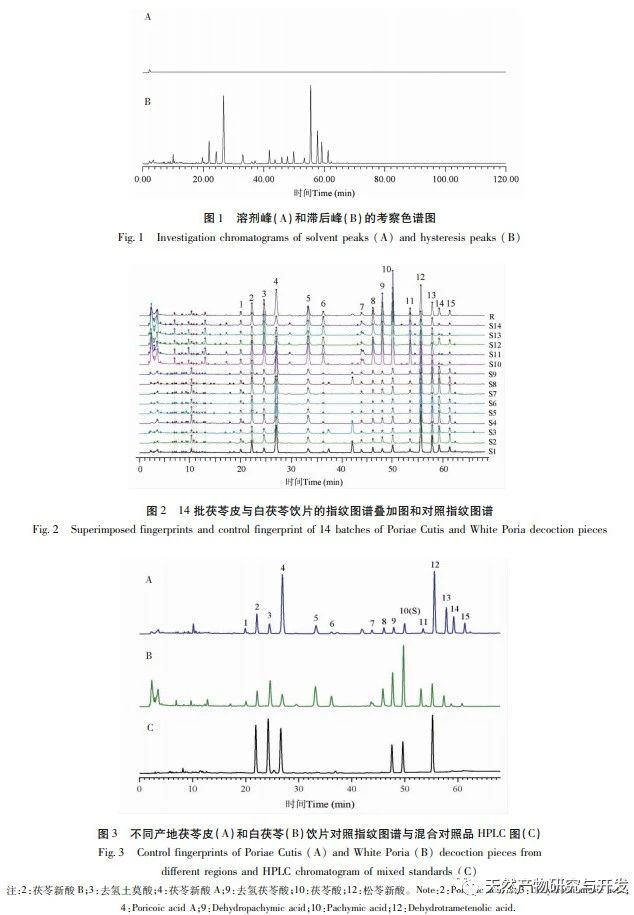
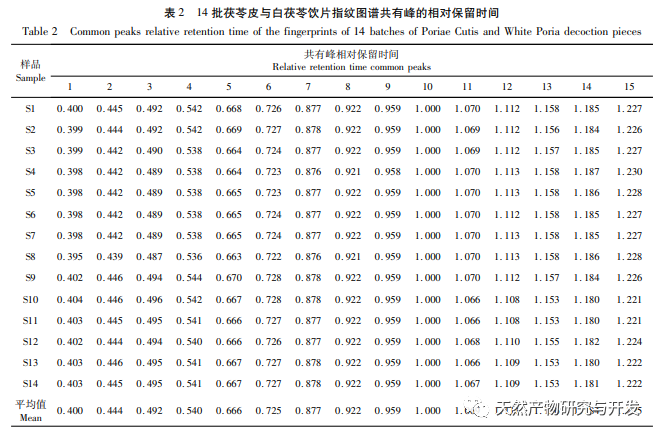
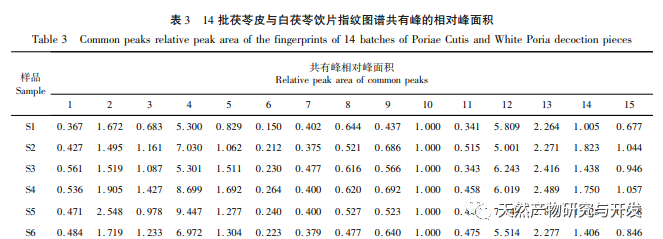
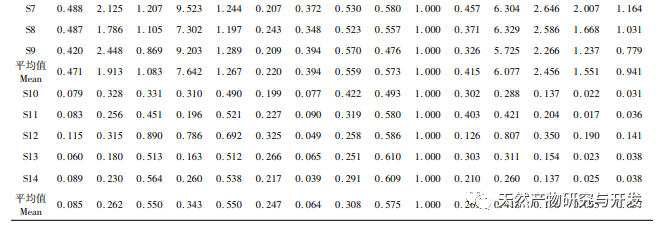
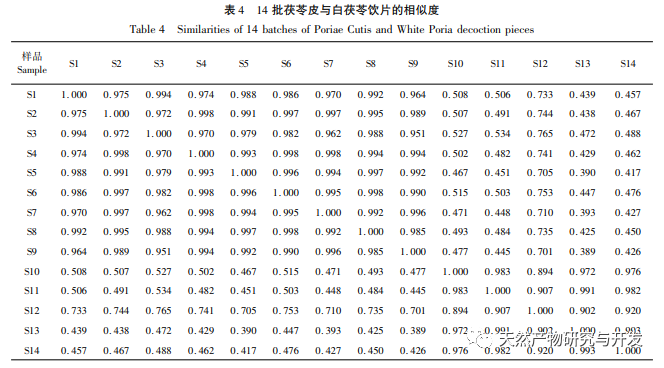
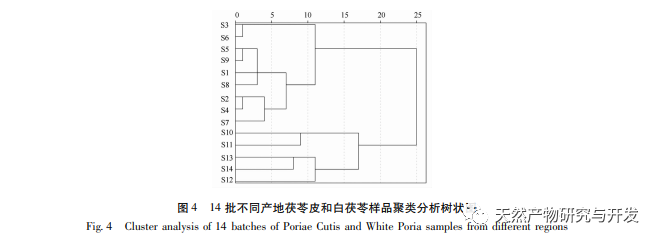
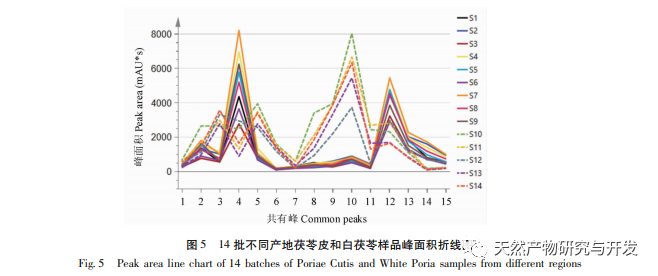
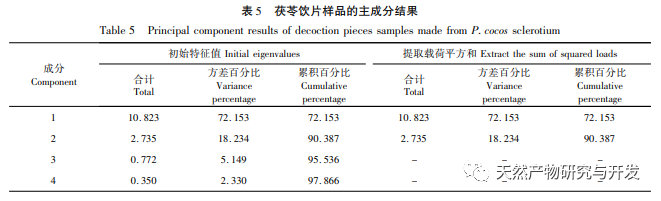
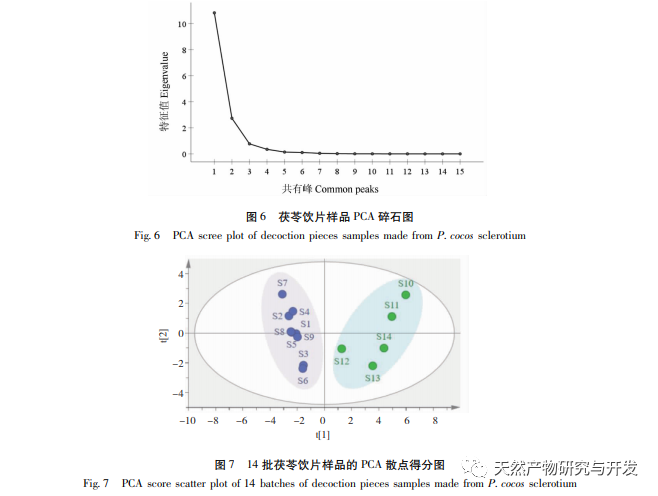
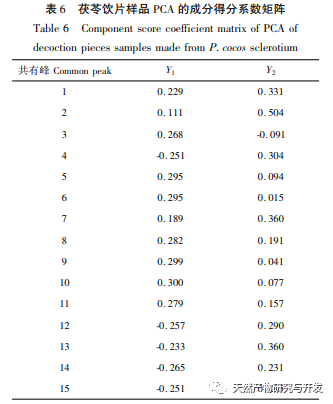
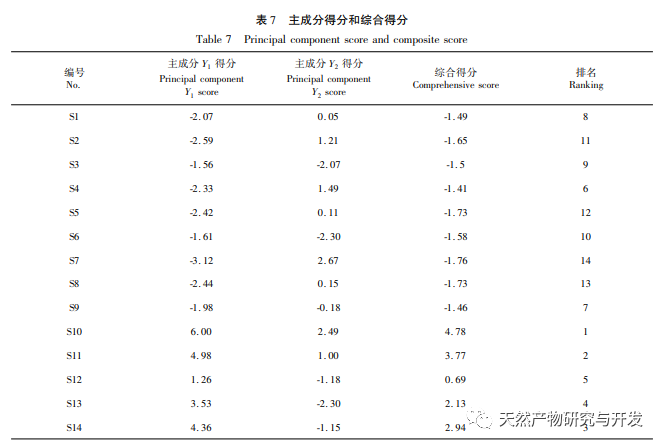
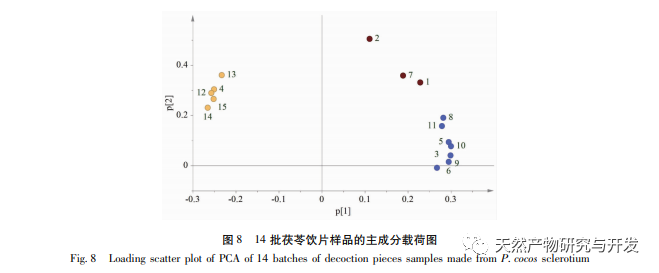
On this basis, HPLC method was used to establish 14 batches of fingerprint spectra of Poria cocos and Poria cocos from different origins. A total of 15 common peaks were calibrated in the shared mode of Poria cocos and Poria cocos fingerprint spectra, and 6 chemical components were identified by chemical reference standards: Poria cocos acid B, dehydrofumaric acid, Poria cocos acid A, dehydrofumaric acid, Poria cocos acid, and Poria cocos acid. Through clustering analysis of 14 batches of Poria cocos skin and white Poria cocos samples from different origins, combined with similarity data, it can be concluded that there are significant differences between groups of Poria cocos samples from different medicinal parts, and small differences within groups. The correlation between white Poria cocos samples and the origin is strong. Therefore, unsupervised PCA method was used for pattern recognition, and the PCA results divided the 15 common peak variables into 2 principal components Y1 and Y2. Y1 is the comprehensive reaction of 7 common chromatographic peaks, including dehydrofumaric acid, dehydroporic acid, and poric acid components, indicating that the 7 common chromatographic peaks are essential for studying the fingerprint spectra of poria cocos skin and white poria cocos; Y2 is a comprehensive reaction of 8 chromatographic peaks, including Poria cocos acid B, Poria cocos acid A, and Poria cocos acid, indicating that these 8 chromatographic peaks can serve as characteristic identification peaks for Poria cocos skin when evaluating the quality of Poria cocos skin and Poria cocos at the same time. The common peak variable parameters that have a significant impact on the fingerprint spectrum are 1, 2 (Poria cocos acid B), and chromatographic peak 7. The difference in content among the 15 common peak components of Poria cocos is smaller than that of Poria cocos skin. In fact, the content of these 15 common peak components in white Poria cocos is much lower than that in Poria cocos skin. In order to make the common pattern of Poria cocos skin and white Poria cocos fingerprint more complete and measurable, a concentration step was added in the preparation of white Poria cocos.
Research has shown that the method established in this article is simple, reliable, and accurate, and can provide reference for the quality evaluation, standard formulation, and rational utilization of Poria cocos and Poria cocos.
