12 New Food Ingredients Approved in 2023
1. Streptomyces pseudoentericus
Approval Announcement: Announcement of the National Health Commission on 28 “Three New Foods” including Leuconostoc pseudomesenteroides (No.1, 2023)
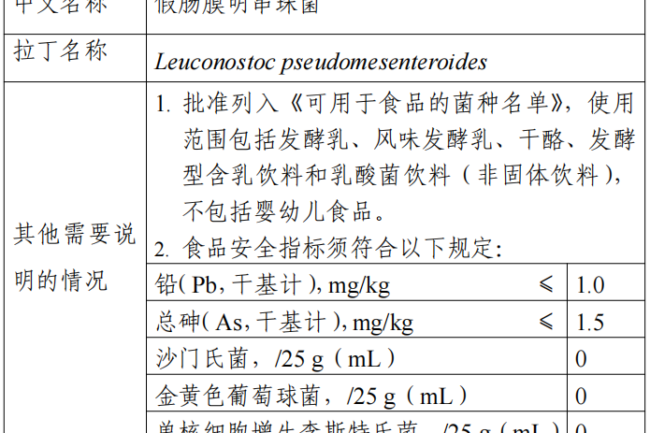
Leuconostoc pseudomesenteroides belongs to the genus Leuconostoc and is isolated from traditional fermented dairy products. This strain has been included in the list of recommended biologics in the European Food Safety Authority (EFSA) QPS list and in the Bulletin of the International Dairy Federation (Bulletin?of?the?IDF?514/2022) “Catalogue of Microbial Species Demonstrated to be Safe in Fermented Foods” and has been approved for use in countries such as Denmark, Canada, and Korea, Korea and other countries have been approved for use.
According to the Food Safety Law of the People’s Republic of China and the Administrative Measures for the Review of the Safety of New Food Ingredients, the National Health and Wellness Commission entrusted the reviewing body to organize experts to review and pass the safety assessment materials of Pseudoenteric Membrane Streptomyces in accordance with the statutory procedures. The production and use of new food ingredients should be in line with the contents of the announcement and the requirements of food safety related regulations.
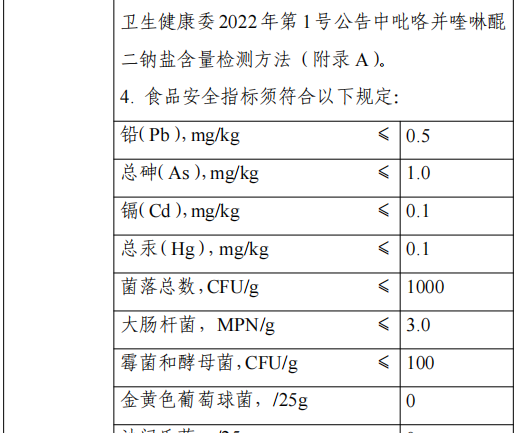
The use of this strain includes fermented milk, flavored fermented milk, cheese, fermented milk beverages and lactobacillus beverages (non-solid beverages), excluding foods for infants and young children. The food safety indicators of this raw material shall comply with the following provisions: lead (as Pb, dry basis) ≤ 1.0 mg/kg, total arsenic (as As, dry basis) ≤ 1.5 mg/kg, microbiological limits for Salmonella 0/25 g (mL), Staphylococcus aureus 0/25 g (mL), Listeria monocytogenes 0/25 g (mL). When the national food safety standards for food processing strain preparations are released, they will be implemented in accordance with the standards for food processing strain preparations.
2.Blueberry anthocyanin
Approval Announcement: Announcement of the National Health Commission on 14 kinds of “Three New Foods” such as blueberry anthocyanin (No. 3, 2023)
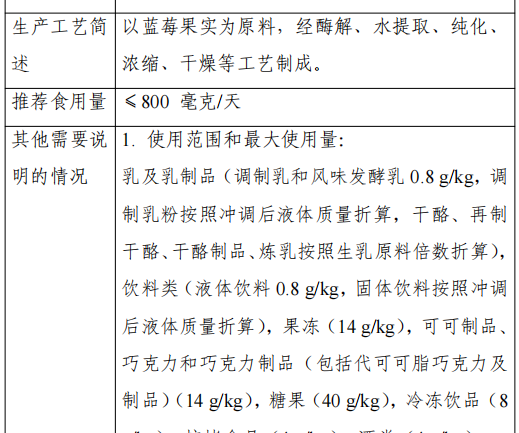
Blueberry anthocyanin is a powdery substance made from the fruit of blueberry (Vaccinium corymbosum?L.) of the family Rhododendron, through the process of enzymatic digestion, water extraction, purification, concentration and drying.
Canada approves the use of blueberry extract (anthocyanin content ≥40%) as natural health food; the EU uses anthocyanin from vegetable and fruit sources as food additives; the U.S. uses anthocyanin from grapes and grape skins sources as a food additive, and allows it to be used in beverages and other foods.
The recommended serving size of this product is: the recommended serving size of blueberry anthocyanoside with 40.0% total anthocyanoside content is 800 mg/day, and those exceeding this content are converted according to the actual content.
According to the “Food Safety Law of the People’s Republic of China” and “Administrative Measures for the Review of the Safety of New Food Ingredients”, the National Health and Health Commission commissioned the review body in accordance with the statutory procedures, the organization of experts on the blueberry anthocyanoside safety assessment of material review and adoption.
The production and use of new food ingredients should be in line with the content of the announcement and the requirements of food safety-related regulations. In view of the lack of information on the safety of blueberry anthocyanin in infants and young children, pregnant women and lactating women, from the principle of risk prevention, the above groups of people should not be consumed, labeling and instructions should be marked inappropriate for the population. The food safety indicators of the raw material in accordance with the announcement.
3. Rye Pollen
Approval Announcement: Announcement of the National Health Commission on 14 kinds of “Three New Foods” such as blueberry anthocyanin (2023 No.3)
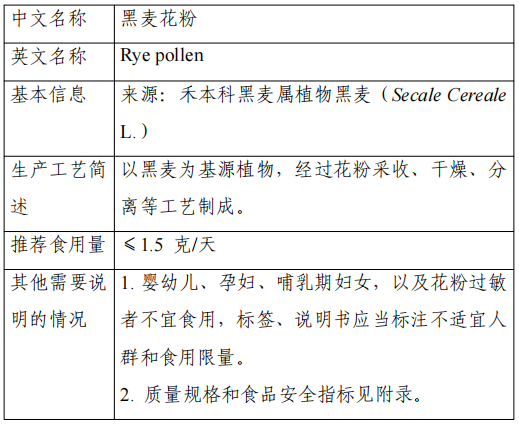
The base plant of this product is rye (Secale Cereale L.) of the grass family, native to Central Asia and the Mediterranean and other regions, and widely cultivated in Europe. This product is made from the pollen of rye, which is harvested, dried and separated.
In Japan and Korea, rye pollen can be consumed as food without limiting its base plant as a food category; in the U.S., rye pollen can be sold as a food ingredient. The recommended serving size of this product is ≤1.5 g/day.
According to the Food Safety Law of the People’s Republic of China and the Administrative Measures for the Review of the Safety of New Food Ingredients, the National Health and Wellness Commission has commissioned the review body to organize experts to review and pass the safety assessment materials of rye pollen in accordance with the statutory procedures.
The production and use of new food ingredients should be in line with the contents of the announcement and the requirements of food safety-related regulations. In view of the insufficient information on the safety of rye pollen in infants and young children, pregnant women and lactating women, from the principle of risk prevention, the above groups should not be consumed, and pollen allergy should not be consumed, labeling and instructions should be marked inappropriate for the crowd. The food safety indicators of the raw material in accordance with the announcement.
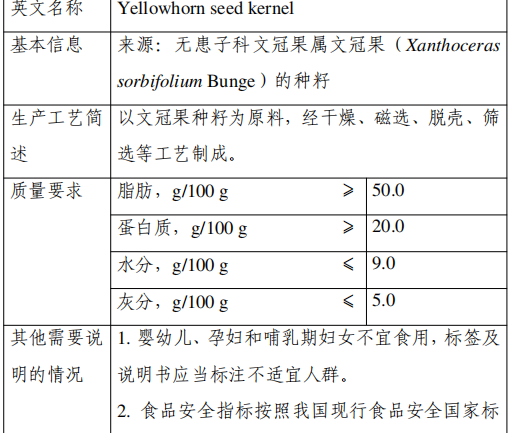
4. Mandarin Seed Kernel
Approval Announcement: Announcement of the National Health Commission on 8 kinds of “Three New Foods”, including the seed kernel of Codonopsis pilosula (No. 5, 2023)
The seed kernel is made from the seeds of Xanthoceras?sorbifolium Bunge of the genus Xanthoceras, family Sapindaceae, through drying, magnetic separation, hulling, screening and other processes.
The main nutrients in the seed kernel of Xanthoceras include fat, protein, carbohydrates, dietary fiber, vitamins, etc., and contains a small amount of saponin and sterols and other substances.
Mandarin has been planted in the northeast, northwest and north of China, and has a history of consumption in Inner Mongolia, Gansu, Shaanxi, Shandong and other regions.
According to the “Food Safety Law of the People’s Republic of China” and “new food ingredients safety review management approach”, the National Health and Health Commission commissioned the review body in accordance with the statutory procedures, the organization of experts on the avena sativa seed kernel of the safety assessment of the material review and through.
The production and use of new food ingredients should be consistent with the content of the announcement and the requirements of food safety-related regulations. In view of the lack of information on the safety of the seed kernels of Avena sativa in infants, young children, pregnant women and lactating women, from the principle of risk prevention, the above groups of people should not be consumed, and labels and instructions should be labeled as inappropriate groups.
The food safety indicators of this raw material in accordance with China’s current national food safety standards in the nuts and seeds of food regulations.
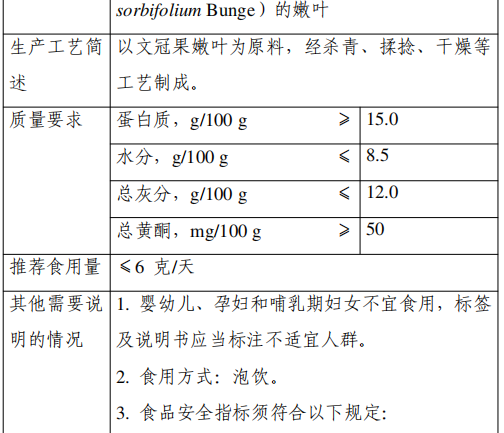
5. Mandarin Leaf
Approval Announcement: Announcement of the National Health Commission on 8 “Three New Foods”, including the seed kernel of Avena sativa (No. 5, 2023)
The leaves of Xanthoceras sorbifolium Bunge are made from the young leaves of Xanthoceras sorbifolium Bunge, family Sapindaceae, through the process of greening, kneading, drying and other processes.
The main nutrients in the leaves of Xanthoceras include carbohydrates, proteins, fats, etc., and contain a small amount of tea polyphenols, polysaccharides, saponins, flavonoids and other substances.
Wenwen is planted in the northeast, northwest and north of China, and the leaves of Wenwen have a history of consumption in Hebei, Shanxi, Inner Mongolia, Shandong and other regions of China. This declared product is consumed in the form of infusion, and the recommended serving size is ≤6g/day.
According to the “Food Safety Law of the People’s Republic of China” and “Administrative Measures for the Review of the Safety of New Food Ingredients”, the National Health and Health Commission commissioned the review body in accordance with the statutory procedures, the organization of experts on the safety assessment of the avena sativa leaf material review and through.
The production and use of new food ingredients should be in line with the content of the announcement and the requirements of food safety-related regulations. In view of the lack of information on the safety of avena sativa in infants and young children, pregnant women and lactating women, from the principle of risk prevention, the above groups should not be consumed, labeling and instructions should be marked inappropriate for the population.
The food safety index of this raw material is implemented according to the announcement. When the national food safety standards for tea substitutes are released, they will be implemented in accordance with the standards for tea substitutes.
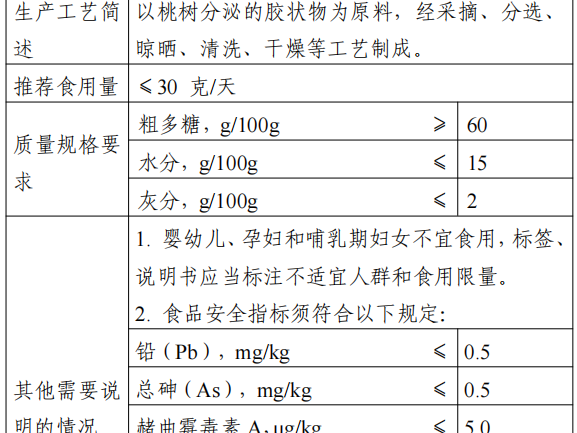
6. Peach Gum
Approval Notice: Interpretation of “on peach pectin and other 15 kinds of” three new food “announcement” (No. 8 of 2023)
Peach Gum is based on the Rosaceae plum plants (Prunuspersica (L.) Batsch) secretion of the gelatinous material as raw material, after picking, sorting, drying, washing, drying and other processes.
The main nutrients are dietary fiber, polysaccharide, water, protein and vitamins. Peach gum in China’s Hubei, Jiangsu and Zhejiang and other regions have a certain history of consumption, consumption is mainly made of soup, porridge, soup, desserts and so on. The recommended serving size of this product is ≤30g/day.
According to “Food Safety Law of the People’s Republic of China” and “new food ingredients safety review management approach”, the National Health and Health Commission commissioned the review body in accordance with the statutory procedures, the organization of experts to review the safety assessment of peach gum materials and through.
The production and use of new food ingredients should be in line with the content of the announcement and the requirements of food safety-related regulations. In view of the lack of information on the safety of peach pectin in infants and young children, pregnant women and lactating women, from the principle of risk prevention, the above groups should not be consumed, labeling and instructions should be marked with unsuitable for the population and consumption limits. The food safety indicators of this raw material in accordance with the provisions of the announcement.
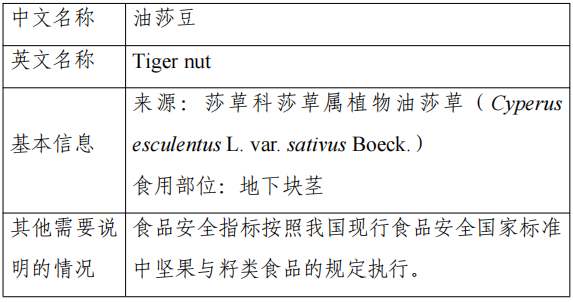
7.Oil Sage Bean
Approval Announcement: Interpretation of “Announcement on Peach Gum and 15 Other ‘Three New Foods’” (No.8 of 2023)
The base plant of this product is Cyperusesculentus L. var. sativusBoeck. The base plant of this product is Cyperusculentus L. var. sativusBoeck. The base plant of this product is Cyperusculentus L. var. sativusBoeck. The base plant of this product is Cyperusculentus L. var. sativusBoeck. The base plant of this product is Cyperusculentus L. var. sativusBoeck.
Declaration of the product oil beans for its underground tubers, the main nutrients for carbohydrates, fat, dietary fiber, water and vitamins and so on. Europe will be oil soybean as common food management; Canada that oil soybean milk has a history of food safety as food consumption.
According to the “Food Safety Law of the People’s Republic of China” and “Management Measures for the Review of the Safety of New Food Ingredients”, the National Health and Health Commission commissioned the review body in accordance with the statutory procedures, the organization of experts on the safety assessment of oilsa bean material review and adoption.
The production and use of new food ingredients should be in line with the contents of the announcement and the requirements of food safety-related regulations. The food safety indicators of this raw material in accordance with China’s current national standards for food safety in the nuts and seeds of food.
8. Intestinal membrane bright Streptomyces lactis subspecies
Approval Announcement: Interpretation of “on peach gum and other 15 kinds of” three new food “announcement” (No. 8 of 2023)
Streptomyces intestinalis Lactobacillus subspecies is mainly found in naturally fermented dairy products, cheese, kimchi, etc. The strain used in this product is derived from Lactobacillus casei. The strain used in this product was isolated from dairy products and is included in the list of recommended biological agents on the European Food Safety Authority’s (EFSA) list of qualifications (QPS), in the Bulletin of the International Dairy Federation (BulletininoftheIDF514/2022), in the “Catalogue of Microbial Species Demonstrated to be Safe in Fermented Foods”, and in the Danish “List of Food Products” (No. 8, 2023). “ and Denmark’s “Record of the List of Microbial Species for Use in Food”.
This approval is included in the “List of strains that can be used in food”, and the scope of use includes the fermentation and processing of milk and dairy products, fruit and vegetable products, and cereal products, excluding baby food.
According to the “Food Safety Law of the People’s Republic of China” and “Management Measures for the Review of the Safety of New Food Ingredients”, the National Health and Health Commission commissioned the review body in accordance with the statutory procedures, and organized experts on the safety assessment of intestinal membranes Ming Streptomyces lactis subspecies of the material for review and adoption.
The production and use of new food ingredients should be in accordance with the contents of the announcement and the requirements of food safety related regulations. When the national food safety standards for strain preparations for food processing are released, they shall be implemented in accordance with the standards for strain preparations for food processing.
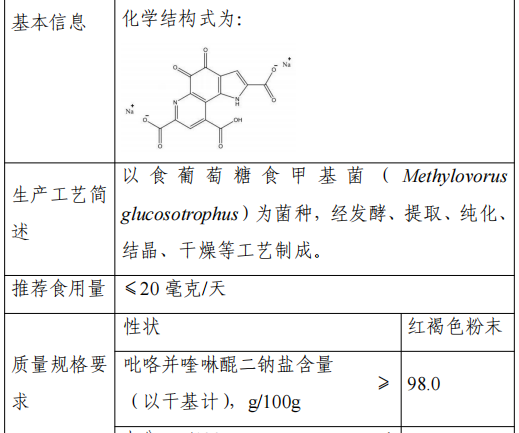
9.Pyrroloquinoline quinone disodium salt
Approval Announcement: Interpretation of “Announcement on Peach Gum and other 15 kinds of ‘Three New Foods’ (No. 8 of 2023)
This product is made from Methylovorusglucosotrophus as fermentation strain, through fermentation, extraction, purification, crystallization, drying and other processes.
Pyrroloquinoline quinone disodium salt is naturally found in many kinds of food such as milk, egg, spinach and so on. We have approved the synthetic method of pyrroloquinoline quinone disodium salt as a new food ingredient in 2022.
Pyrroloquinoline quinone disodium salt is regulated as “Generally Recognized As Safe (GRAS)” in the United States, and can be used as an ingredient in energy drinks, sports drinks, electrolyte drinks and other food products; it is used in the European Union and Canada as a dietary supplement or natural health food.
The recommended serving size of this product is ≤20 mg/day (i.e., the recommended serving size of pyrroloquinoline quinone disodium salt with 98% content is ≤20 mg/day, and those exceeding this content are converted according to the actual content).
According to the provisions of the Food Safety Law and the Administrative Measures for the Review of the Safety of New Food Ingredients, the National Health and Health Commission entrusted the review body to organize experts to review and pass the safety assessment materials of pyrroloquinoline quinone disodium salt in accordance with the statutory procedures.
The production and use of new food ingredients should be consistent with the content of the announcement and the requirements of food safety-related regulations. In view of the insufficient information on the safety of pyrroloquinoline quinone disodium salt in the population of infants and young children, pregnant women and lactating women, from the consideration of the principle of risk prevention, it is not suitable for consumption by the above mentioned groups, and the label and instructions should be labeled with the inappropriate groups and the consumption limit. The food safety indexes of this raw material are in accordance with the announcement.
10.Paraguay Holly Leaf (Matei Tea Leaf)
Approval Announcement: Interpretation of the Announcement on Paraguayan Holly Leaves (Matei Tea Leaves) and 9 Other “Three New Foods” (No.10 of 2023)
Paraguayan Holly Leaves (Matei Tea Leaves) is made from the leaves of Paraguayan Holly (Ilex?paraguariensis?A.St.-Hil.), a plant of the genus Holly, in the family of Holly, through the process of picking, roasting, chopping and drying. The main nutrients are carbohydrates, crude fiber, protein, fat, vitamins, minerals and amino acids, and contains a small amount of polyphenols, flavonoids and saponins and other substances.
Paraguayan holly leaves (mate leaves) are regulated as “Generally Recognized As Safe (GRAS)” in the United States, approved for use as a new food ingredient in the European Union, approved for use as a natural health food in Canada, and approved for use in tea production in Brazil.
According to the provisions of the Food Safety Law of the People’s Republic of China and the Administrative Measures for the Review of the Safety of New Food Ingredients, the National Health and Wellness Commission entrusted the review body to organize experts to review and pass the safety assessment materials of Paraguayan holly leaves (mate tea leaves) in accordance with the statutory procedures.
The production and use of new food ingredients should be in line with the content of the announcement and the requirements of food safety-related regulations. In view of the insufficient information on the safety of consumption of Paraguayan wintergreen leaves (Matei Tea Leaves) in infants and young children, pregnant women and lactating women, from the principle of risk prevention, the above groups should not be consumed, and labels and instructions should be labeled with inappropriate groups.
The food safety index of this raw material is implemented in accordance with the announcement. When the national food safety standards for tea substitutes are released, they will be implemented according to the standards for tea substitutes.
11. Yeast Protein
Approval Announcement: Interpretation of the Announcement on 9 “Three New Foods”, including Paraguayan Holly Leaf (Matei Tea Leaf) (No. 10, 2023)
Yeast protein is made from brewer’s yeast (Saccharomyces?Cerevisiae) as a strain, which is collected after cultivation, fermentation and centrifugation to obtain the raw material of the bacterium, and is made by removing nucleic acid, centrifugation, enzymolysis, extraction, purification, isolation, sterilizing and drying, etc. The main nutrient content is protein (protein).
The main nutrients are protein (≥70.0g/100g), fat, dietary fiber and water. At present, the United States has approved brewer’s yeast protein as a nutritional supplement added to food, and the European Union has approved brewer’s yeast protein as a new food ingredient, both of which have not been limited to the amount of consumption.
According to the “Food Safety Law of the People’s Republic of China” and “Management Measures for the Review of the Safety of New Food Ingredients”, the National Health and Health Commission commissioned the review body in accordance with the statutory procedures, the organization of experts on the safety assessment of yeast protein material review and adoption.
The production and use of new food ingredients should be in line with the contents of the announcement and the requirements of food safety-related regulations. In view of the lack of information on the safety of yeast protein in infants and young children, pregnant women and lactating women, from the principle of risk prevention, the above groups should not be consumed, labeling and instructions should be marked inappropriate for the population. The food safety indicators of this raw material in accordance with the announcement.
11.Catechin
Approval Announcement: Interpretation of the Announcement on 9 “Three New Foods” including Paraguayan Holly Leaf (Maté Tea Leaf) (No.10 of 2023)
Catechin is made from tea as raw material, through alcohol extraction, concentration, separation, extraction, enzymatic digestion, concentration, drying and other processes.
The main components are catechins, including epicatechin (EC), epigallocatechin (EGC), hydrated epicatechin gallate (ECG-H2O), hydrated epigallocatechin gallate (EGCG-H2O), gallocatechin gallate (GCG), catechin (dl-C), the total content of catechin (on a dry basis) ≥90?g/100g, of which EGCG content is ≥90?g/100g, of which EGCG content is ≥90?g/100g. 100g, of which EGCG content ≥ 50?g/100g.
The former Ministry of Health approved epigallocatechin gallate (EGCG) as a new resource food in Announcement No. 17 of 2010, with a recommended daily consumption of ≤300 mg/day (in terms of EGCG).
Green tea catechins have been approved by Japan as functional ingredients for specific health foods. The recommended daily intake of this product is ≤300 mg/day (in terms of total catechins) (i.e. the recommended daily intake of raw materials with total catechin content of 100?g/100g is ≤300 mg/day, and the content of 90-100?g/100g is converted according to the actual content).
According to the “Food Safety Law of the People’s Republic of China” and “Administrative Measures for the Review of the Safety of New Food Ingredients”, the National Health and Health Commission commissioned the review body in accordance with the statutory procedures, the organization of experts on the safety assessment of catechins material review and adoption. The production and use of new food ingredients should be in line with the content of the announcement and the requirements of food safety-related regulations.
In view of the lack of information on the safety of catechins in infants and young children, pregnant women and lactating women in the population, from the principle of risk prevention, the above groups should not be consumed, labeling and instructions should be marked unsuitable for the population and consumption limits. The food safety indicators of this raw material in accordance with the provisions of the announcement.