The approval of new food ingredients has always been a hot information that companies are very concerned about, the announcement of new food ingredients means that a certain or some new food ingredients have passed the safety review, the application of China’s legalized, may quickly break out of the listing of a certain food boom. However, in the actual application of new food ingredients, we may have some questions about the approval of new food ingredients, the use and labeling, the following food partner network on some common problems to explore the instructions.
New food ingredients definition, management and query
First, what is new food ingredients
With the change of China’s new food ingredients management system, the name of new food ingredients from the “new resource foods” to “new food ingredients” in the process of change, the connotation of the concept of new food ingredients has also changed.
The current “Administrative Measures for the Review of the Safety of New Food Ingredients” clearly states: “New food ingredients refer to the following items that are not traditionally consumed in China:
(a) Animals, plants and microorganisms;
(ii) Components isolated from animals, plants and microorganisms;
(iii) Food ingredients whose original structure has been changed;
(d) Other newly developed food ingredients.” Which “traditional eating habits” refers to a food in the provincial jurisdiction has more than 30 years as a stereotyped or non-stereotyped food production and management of the history of packaging, and is not included in the “Pharmacopoeia of the People’s Republic of China”.
Second, the approval of new food ingredients management
With the characteristics of food ingredients, in line with the nutritional requirements should have, and non-toxic, harmless, does not cause any acute, subacute, chronic or other potential hazards to human health, and in line with the above definition of new food ingredients, such as the need to develop for the production and operation of ordinary food, should be in accordance with the “safety of new food ingredients review of the management approach” provisions of the declaration of approval.
However, those that have been included in the “National Standard for Food Safety, Standard for Use of Food Additives” (GB 2760) and “National Standard for Food Safety, Standard for Use of Nutritional Enrichment” (GB 14880) are not included in the scope of declaration.
According to the requirements of the Food Safety Law, the use of new food raw materials for the production of food should be submitted to the health administrative department of the State Council (the National Health and Wellness Commission) to assess the safety of the relevant products, and the health administrative department of the State Council will organize the review.
Applicants in accordance with the “Declaration and Acceptance of New Food Ingredients Regulations” and other regulatory requirements, to the National Health and Health Commission to submit the required declaration materials, the National Health and Health Commission Hall of Government on the receipt of application materials for review, and depending on the review to make the acceptance, inadmissibility, make corrections to the materials of the decision letter or notice. If the public wants to know the new food ingredient approval dynamics, they can query through the government service platform of the National Health Commission: https://zwfw.nhc.gov.cn/kzx/sdxx/sdxxqb/.
The Expert Review Committee makes technical evaluation conclusions on new food ingredients through evaluation. The final approval results are divided into three kinds: approval announcement, not licensed, termination of the review.
III. Explanation on Termination of Review
In the approval process of new food ingredients, there are three cases of expert review committee to make “termination of the review” of the technical assessment conclusions: after review of common food or common food with substantial equivalence; and has been announced with the new food ingredients with substantial equivalence; and other cases to terminate the review. Substantial equivalence means that if a newly declared food ingredient is the same as a food or an announced new food ingredient in terms of species, source, biological characteristics, main ingredients, food parts, usage amount, scope of use and application population, etc., and the process and quality requirements adopted are basically the same, it can be regarded that they are equally safe and have substantial equivalence. The following are examples for the above termination of review.
(1) Reviewed as common food or substantially equivalent to common food: soybean oligosaccharide, the termination of the review opinion is “there are national standards for soybean oligosaccharide, when used as food raw materials, it should be implemented according to the relevant content of the standard for soybean oligosaccharide (GB/T22491-2008).”
(2) Substantial equivalence with the announced new food ingredient: Sodium Hyaluronate, with the termination opinion as “This product is produced by fermentation of Streptococcus equi subspecies of Streptococcus equi with glucose, yeast powder, peptone and so on as the culture medium. It is substantially equivalent to Sodium Hyaluronate (former Ministry of Health Announcement No. 9 of 2020), which has been approved. It is recommended that the review be terminated and implemented in accordance with the relevant content of the published sodium hyaluronate.” Sodium hyaluronate that conforms to the review opinion is managed as a new food ingredient.
(3) Other cases of termination of the review: Lotus corniculatus, the opinion of termination of the review is “In view of the fact that Lotus corniculatus has been included in the Pharmacopoeia of the People’s Republic of China in a variety of single and prescription preparations, with clear pharmacological activity, it is recommended to terminate the review.” It can be clear that goldenseal is not a food ingredient.
Fourth, the query of new food ingredients
If you want to know whether a certain ingredient is an approved new food ingredient, or you want to check what new food ingredients are currently approved, terminated for review or open for comments, you can query through the following ways.
(1) Food Partner’s Food Ingredients Information Database
The Food Ingredients Information Database of FoodPartner.com summarizes the information on new food ingredients approved and terminated for review over the years, and associates the original announcement of new food ingredients and the related reply letters, which is a very convenient query tool. Inquiry link: http://db.foodmate.net/xinshipin/.
(2) Public Query System for Health Administrative License of the National Health and Wellness Commission
In this query system, it is divided into the directory of new resource foods approved before 2007 and the directory of new food ingredient announcements approved after 2007, and the directory of termination review of new food ingredients. Inquiry link: https://slps.jdzx.net.cn/xwfb/gzcx/PassFileQuery.jsp.
(3) Official website of the National Health and Wellness Commission
If you want to check the approval status and announcement details of a certain new food ingredient, you can also search the name of the new food ingredient directly on the official website of the NHRC.
(4) National Risk Assessment Center Administrative License Request for Comments
If you want to know the information about the new food ingredient request for comments, you can use the administrative license request for comments section of the Risk Assessment Center to learn about the relevant developments. Inquiry link: https://www.cfsa.net.cn/Article/LawNew_List.aspx?channelcode=FD7DFE7A58DAB7788ED6929809972C8AE0FC102162B069D1&code= 9535ed5882d69ad7f633fea0205be83a.
Fully understand the content of the announcement of new food ingredients
and the compliant use of new food ingredients
I. Pay attention to the announcement content of new food ingredients
According to the “Administrative Measures for the Review of the Safety of New Food Ingredients”, the announcement of new food ingredients usually contains the name of the raw material, source, production process, main ingredients, quality specifications, labeling requirements and other content to be announced. Among them, “other information to be announced” is very changeable, about the unsuitable people and special labeling requirements are usually included in this element.
When we decide whether a certain raw material is a new food ingredient, we need to search the announcement of new food ingredients, carefully review the original announcement, and fully understand the content of the announcement, including food parts, food consumption, food consumption, production process, quality specifications, scope of use, unsuitable groups, etc., to avoid misuse of new food ingredients.
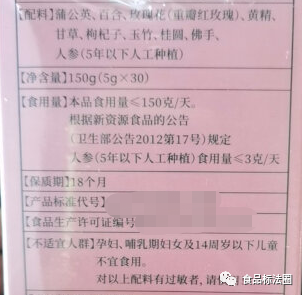
For example, “Ginseng (artificial planting)”, in the basic information column of the announcement of the new food ingredient, it is clear: “Source: ginseng planted artificially for 5 years and less than 5 years”, which means that ginseng planted artificially for more than 5 years is not in the scope of approved new food ingredients. Another example is “Mujing Ye Ke”, which is clearly stated in the announcement that the edible part is young leaves (buds) and the way of consumption is brewing, so other parts that are not within the scope of the announcement, or the way of consumption is different from the announcement, also do not belong to the scope of the announcement of the new food ingredient.
Announcement of new food ingredients in most cases, will indicate the Latin name of the raw material, can not tell whether the raw material used in the announcement of the raw material, you can verify the Latin name of the two are consistent to assist in judgment.
Second, the new food ingredients concentration conversion
Some new food ingredients will be microencapsulation and dilution process to produce a lower concentration of new food ingredients announced, in this case, the consumption of new food ingredients need to be calculated according to the concentration of the product equivalent.
In this case, reference is made to the announcement of the former Ministry of Health on the approval of lutein esters and the reply letter on the issue of the use of lutein esters at low concentration: “Announcement of the Ministry of Health on the Approval of 7 New Resource Foods, including Lactobacillus Acidophilus” (No.12, 2008), which requires that the lutein dipalmitate content of lutein esters be >55.8%, and that the consumption amount be less than ≤12mg/d; the “Reply Letter of the General Office of the Ministry of Health on the Issues of the Use of Lutein Esters” clarifies that: “The consumption amount of the new food ingredient should be calculated according to the concentration of the product. The Reply Letter of the General Office of the Ministry of Health on the Use of Lutein Esters” clarifies that “the low concentration of lutein esters produced by microencapsulation and dilution process can be used as food raw materials, and its consumption should be calculated according to the concentration of the product”.
Based on the above, assuming that the content of lutein dipalmitate in the diluted lutein ester is 20%, its consumption should be <(55.8%×12/20%)(mg/day).
III. Compliance of Bringing in New Food Ingredients
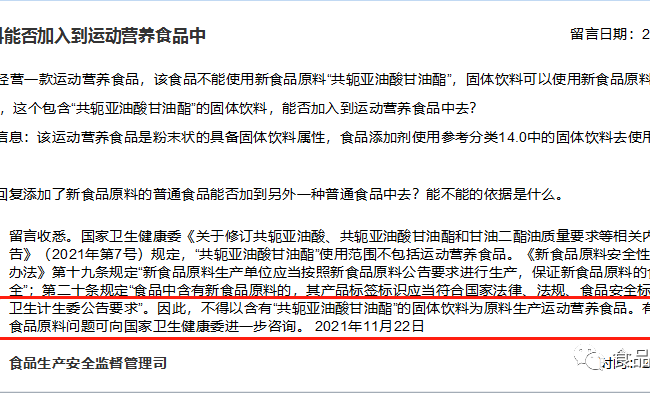
In November 2021, the State Administration for Market Supervision and Regulation (SAMSAR) responded to a public message on “whether solid drinks can be added to sports nutrition food”, which attracted attention, and the original text is shown below. Through the message and the response, it can be seen that the attitude of the market supervision: it does not support the intentional use of specific raw materials to bring a new food ingredient that cannot be used in the final product into the final product.
How to Label New Food Ingredients in the List of Ingredients
I. Labeling Requirements for New Food Ingredients in the Ingredient List
Administrative Measures for the Review of the Safety of New Food Ingredients” clearly: “Food containing new food ingredients, its product labeling should be consistent with national laws, regulations, food safety standards and the National Health and Family Planning Commission announcement requirements.” According to this provision, the names of new food ingredients in the food ingredient list should also comply with the requirements of the announcement.
Failure to label the names of new food ingredients in strict accordance with the requirements of the announcement may be misleading. Take “ginseng” as an example, “ginseng” is included in the “List of articles that can be used in health food”, and the articles listed in the list are limited to be used in health food; the former Ministry of Health Announcement No. 17 of 2012 specifies that “ginseng (artificially grown)” is included in the list of new food ingredients. Ginseng (Artificial)” is included in the management of new food raw materials. Therefore, it is inaccurate to label “ginseng” directly in the ingredient list, and when it is applied to food as a new food ingredient, it should be labeled with the name “ginseng (artificial cultivation)” in the announcement.
Labeling of serving size of new food ingredients
According to the “National Health and Family Planning Commission Food Division on the prepackaged food containing new food ingredients labeling and oligofructose related issues of the reply” (National Health Food Labeling Letter [2015] No. 279): “According to the “National Food Safety Standards General Rules for the Labeling of Prepackaged Foods” (GB 7718-2011) relevant provisions, the method of consumption belongs to the recommended labeling content. consumption method belongs to the recommended labeling content.
If the prepackaged food contains new food ingredients that have been announced, if the announcement explicitly requires the labeling of the serving size and unsuitable population in the labeling and instructions, the labeling should be made in accordance with the requirements of the relevant announcement; if the announcement has the requirements of the serving size and unsuitable population but does not require the labeling of them in the labeling and instructions, they can be labeled or not at the discretion of the food production enterprises.”
The former Ministry of Health Announcement No. 12 of 2008 specifies that the serving size of Aloe vera gel should be ≤30g/day; however, the announcement does not require that the “serving size” be indicated on the label of the product.
In the Announcement of the Ministry of Health and other 6 Ministries and Bureaus on the Labeling Requirements for Foods Containing Aloe Vera Gel (Announcement No. 1 of 2009), there is a corresponding explanation on the labeling of the serving size of Aloe Vera Gel: “Enterprises should stipulate the daily serving size of food products with added Aloe Vera Gel in the enterprise standards. If it is not possible to ensure that the daily intake of aloe vera by consumers is within the safe range, a daily serving size warning should be labeled on the package.”
Combining the above reply letter and the announcement requirements, it can be seen that if the new food ingredient announcement does not explicitly require the labeling of “serving size”, it can be combined with the consumption requirements of the announcement and the final consumption of the food to determine whether or not to indicate the “serving size” on the label. “The label can be determined by taking into account the serving size requirement of the announcement and the final consumption of the food. Can ensure that the daily intake of consumers in the safe range, you can choose whether to label “serving size”, can not ensure that the daily intake of new food ingredients in the safe range of consumers, it is recommended to label the daily serving size on the package.
Third, the labeling of unsuitable people
Some new food ingredients in the declaration of infants and young children, pregnant women and other special populations did not provide safety assessment of food materials, or materials provided are not sufficient to ensure the safety of consumption in special groups, therefore, the announcement of its new food ingredients expressly required to indicate the inappropriate population, such as “BaoLeGuo powder”, “rice bran fatty alcohols”, “ginseng (cultivated)”, etc. If such new food ingredients are added to the final product, the labeling should indicate the unsuitable population according to the requirements.
Examples of the above labeling of name, serving size and unsuitable population in the ingredient list are shown below.