Study on the inhibitory effect of lotus leaf alkaloid on Akt/mTOR/4EBP1 glycolysis pathway and its anti proliferative effect on cholangiocarcinoma cells
Cholangiocarcinoma (CCA) is divided into extrahepatic cholangiocarcinoma (extrahepatic cholangiocarcinoma) and intrahepatic cholangiocarcinoma (ICC) based on anatomical location. Although ICC accounts for only 10% to 20% of primary liver cancer, it is highly insidious in the early stages, with a high postoperative recurrence rate and poor prognosis. In recent decades, the incidence rate and mortality of ICC in Asia and even the world have been rising steadily. The first-line chemotherapy program for advanced ICC is prone to drug resistance and adverse drug reactions after long-term use, so it is necessary to explore safe and effective therapeutic drugs.
The screening of natural medicinal active ingredients with anti-tumor effects from plants and animals has always been a research hotspot both domestically and internationally. Nuciferine (NUF) originates from the dried leaves of Nelumbo nucifera Gaertn, a plant in the Nymphaeaceae family. It has certain anti-tumor cell activity, but its effect on ICC cells is rarely reported. This study investigated the effects of lotus leaf alkaloid on the proliferation and glycolysis process of HuCCT1 cells, and preliminarily explored its mechanism of action, providing a theoretical basis for the development of safe and effective ICC therapeutic drugs.
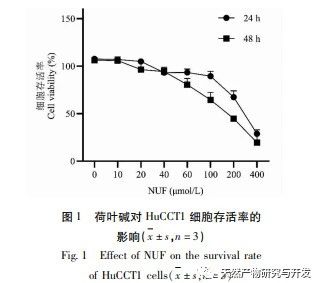
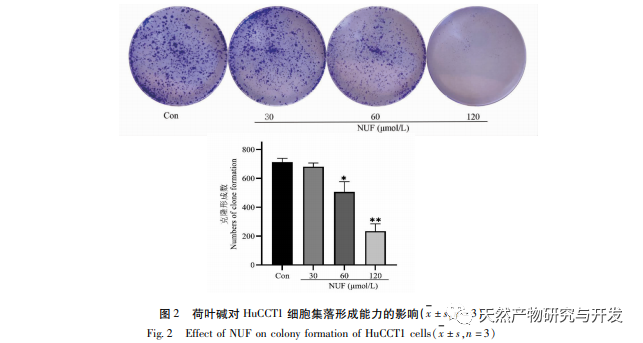
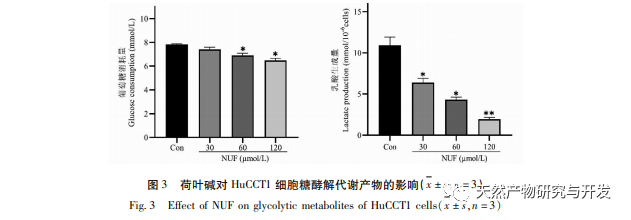
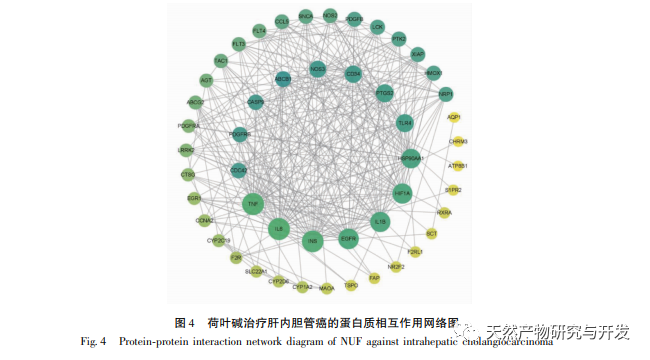
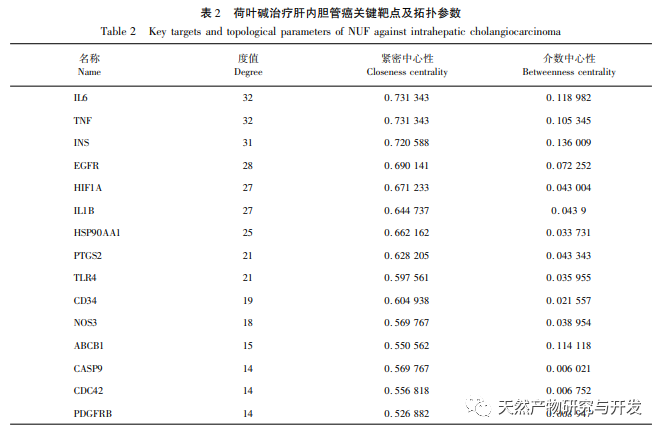
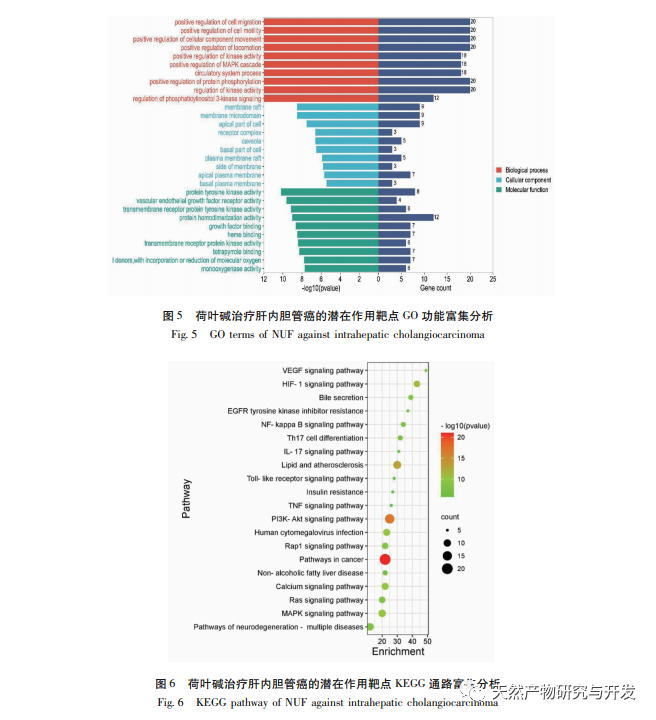
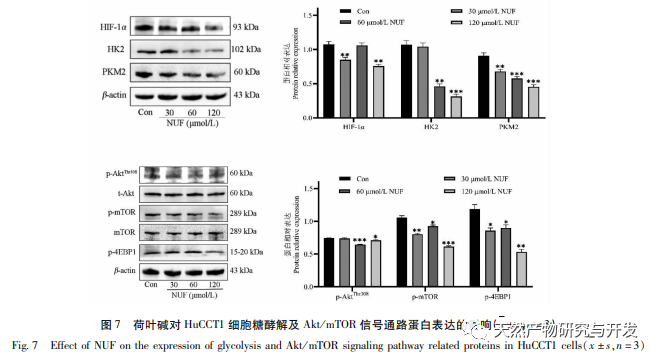
Modern pharmacology research shows that the active component of lotus leaf alkaloid extracted from lotus leaf has certain anti growth effect on many tumor cells, such as liver cancer, lung cancer, breast cancer, and can improve its sensitivity to chemotherapy drugs. This study found that a certain concentration of lotus leaf alkaloid can significantly inhibit the proliferation of HuCCT1 cells in vitro, and is positively correlated with reducing glucose consumption and lactate production. Carbohydrates are important energy sources in cellular life activities, and the Warburg effect indicates that even under normal oxygen conditions, cancer cells preferentially supply energy through glycolysis rather than aerobic metabolic pathways. High levels of glycolysis can provide rapid energy for the growth of cancer cells, and its products such as pyruvic acid and lactic acid also provide raw materials for the synthesis of cancer cell substances and promote cancer cell metastasis and invasion. Therefore, it is speculated that the inhibition of HuCCT1 cell proliferation by lotus leaf alkaloids is related to their inhibition of cellular glycolysis metabolism.
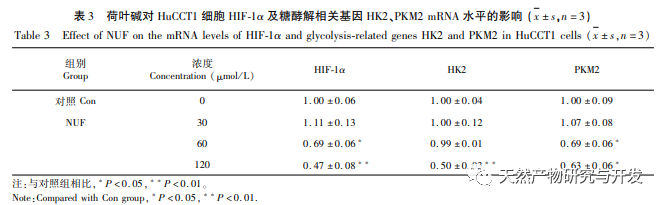
Network pharmacology analysis found a high correlation between matrine and HIF-1 α, as well as targets such as TNF, INS, EGFR, etc. HIF-1 α is involved in multiple processes such as glucose metabolism, cell proliferation, invasion and metastasis, and inflammatory response in tumor cells. It can activate the activity of glucose transporter (GLUT) and various glycolytic enzymes such as HK2, PKM2, and pyruvate dehydrogenase kinase 1 (PDK1), and directly participate in the glycolysis process, greatly promoting tumor progression. Hexokinase (HK) and pyruvate kinase (PK) are two key kinases in the process of glycolysis, regulating the initial and final stages of the process, respectively. This study found that the mRNA levels and protein expression of HIF-1 α and key glycolytic enzymes HK2 and PKM2 in cells gradually decreased with the increase of lotus leaf alkaloid concentration. Therefore, it is speculated that lotus leaf alkaloid may affect the glycolysis process of HuCCT1 cells by reducing the activity of HIF-1 α.
HIF-1 is a type of transcription factor that cells respond to in low oxygen or hypoxic environments, consisting of two subunits: HIF-1 α and HIF-1 β. The proline residue of HIF-1 α is susceptible to hydroxylation under normoxic conditions and binds to its tuning molecule (von Hippel Lindau, pVHL), which is then degraded by ubiquitination. In a low oxygen environment, the activity of prolyl hydroxylase domain containing proteins (PHD) decreases, leading to a significant accumulation of HIF-1 α, which then migrates to the nucleus to form a dimer with HIF-1 β. The dimer then binds to the hypoxic response element (HRE) to promote transcription of HIF-1 target genes. In addition, HIF-1 α can be activated in an oxygen independent manner, for example, the PI3K/Akt/mTOR signaling cascade can upregulate the transcription and translation of HIF-1 α by disrupting the integrity of eukaryotic translation initiation factor 4E (eIF4E) binding protein 1 (4EBP1). Some scholars refer to the activation mechanism of this oxygen independent HIF-1 α signal as “pseudo hypoxia”. In this study, KEGG analysis revealed that matrine acts on the potential pathways of ICC, with a significant increase in the PI3K/Akt signaling pathway. Immunoblotting revealed that matrine can reduce the phosphorylation levels of mTOR and 4EBP1 proteins in HuCCT1 cells, and the overall p-AktTrr308 protein showed a decreasing trend, suggesting that the downregulation of HIF-1 α transcription and translation levels by matrine may be related to the inhibition of the Akt/mTOR/4EBP1 signaling pathway.
Multiple studies have shown that in addition to inducing apoptosis of tumor cells in vitro, inhibiting invasion and metastasis, and blocking them to different cycles, lotus leaf alkaloids can also delay the growth of ectopic transplanted tumors in nude mice and exert significant anti-tumor effects. The main mechanism is related to the regulation of cell cycle and apoptosis related protein expression by lotus leaf alkaloids, direct targeting of specific genes, inhibition of signaling pathways such as PI3K Akt, Wnt/β – catenin, SOX2 Akt, STAT3, and TLR4/NF – κ B. This study preliminarily found that lotus leaf alkaloids inhibit the proliferation of intrahepatic cholangiocarcinoma cells, possibly by suppressing the cellular glycolysis process regulated by the Akt/mTOR/4EBP1 signaling pathway. The intrinsic logical relationship between the two will be explored in the next step of research, in order to elucidate the anti ICC mechanism of lotus leaf alkaloids and provide a theoretical basis for the deep development of lotus leaf resources and clinical applications.
