Study on the transfer law of dosage values between half lotus decoction pieces and standard decoction based on HPLC fingerprint and content determination
Lobelia chinensis Lour., a dried whole plant of the Campanulaceae family, has the effects of clearing heat, detoxifying, diuresis, and reducing swelling. Research has shown that half lotus mainly contains flavonoids, alkaloids, and coumarins, which have pharmacological effects such as antibacterial, antiviral, anti-tumor, anti-inflammatory, and analgesic effects. Half lotus is a commonly used traditional Chinese medicine in pharmacopoeia, but the quality standards for half lotus medicinal materials under pharmacopoeia only include characteristics, identification, inspection, and extraction items, and the quality standards are not yet complete. At present, there are still relatively few reports on the quality control research and component analysis of half lotus.
Traditional Chinese medicine mainly uses decoctions as the main form of medication, which are the material basis for the efficacy of Chinese medicine. After thousands of years of clinical practice, establishing quality standards for Chinese medicine standard decoctions can provide important references for the establishment of quality standards for modern Chinese medicine formulations such as Chinese medicine formula granules. At present, there are no research reports on the standard decoction of half lotus. The author of this article studied the national standard for Banbianlian formula granules according to the “Technical Requirements for Quality Control and Standard Development of Traditional Chinese Medicine Formula Granules” (hereinafter referred to as the “Technical Requirements”), and studied the key quality indicators of the standard decoction: ointment yield, extract, content and transfer rate of indicator components, and fingerprint spectrum. The study examined the transfer law of the quantity value from Banbianlian decoction pieces to the standard decoction, providing reference for the development of quality standards for Banbianlian formula granules and related preparations.

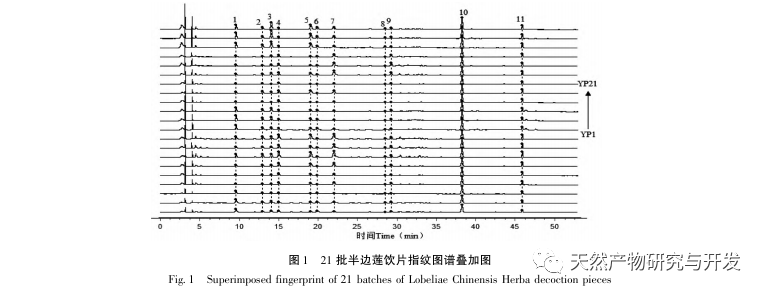
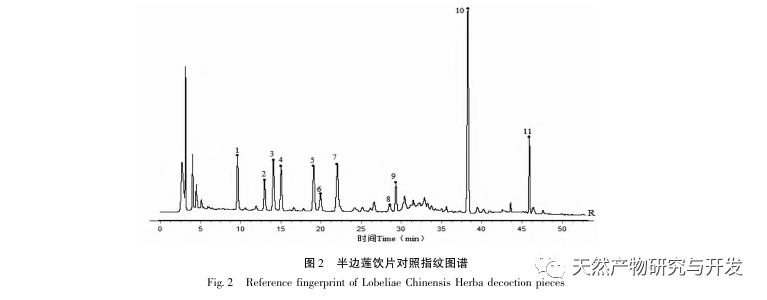
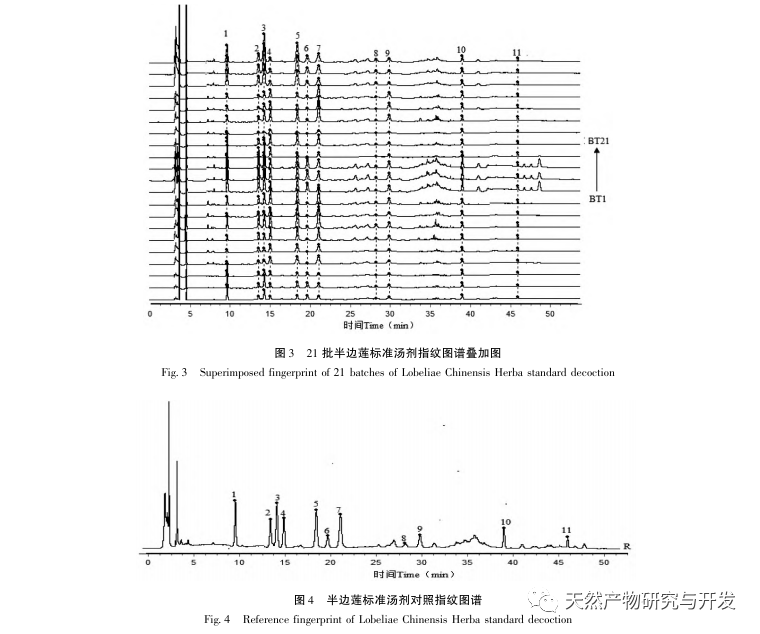
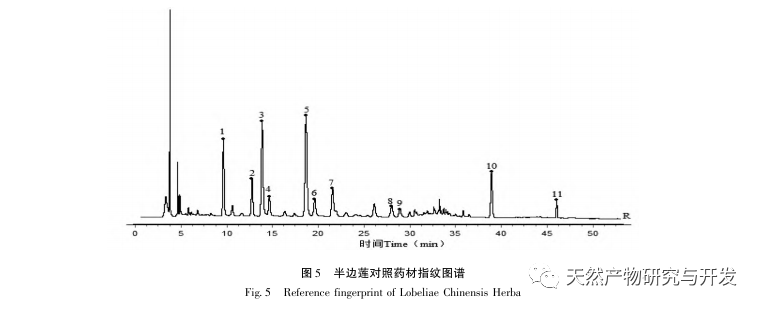
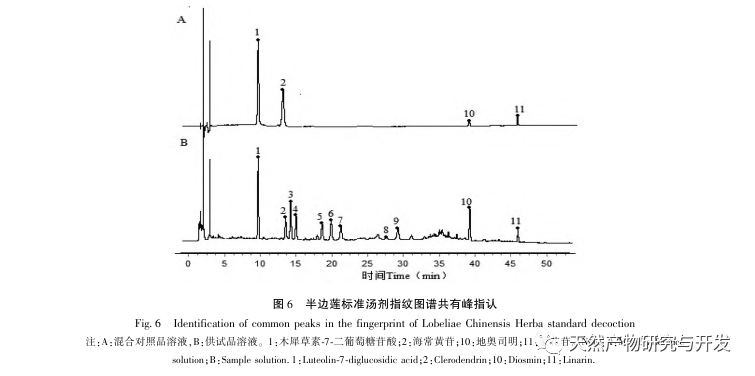
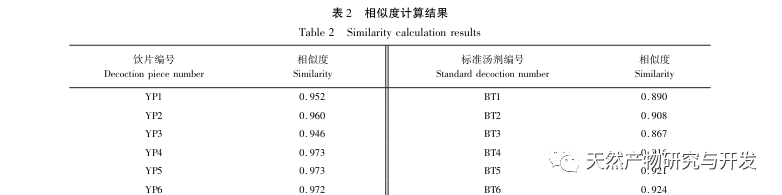
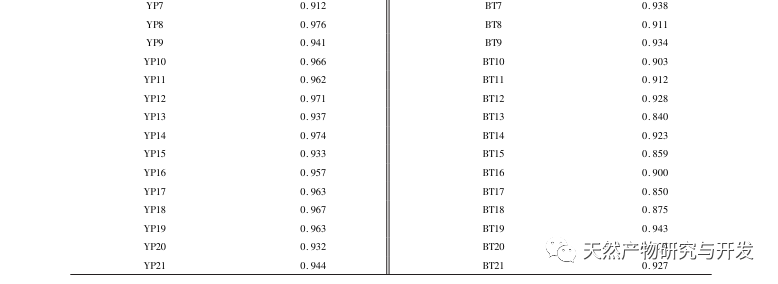
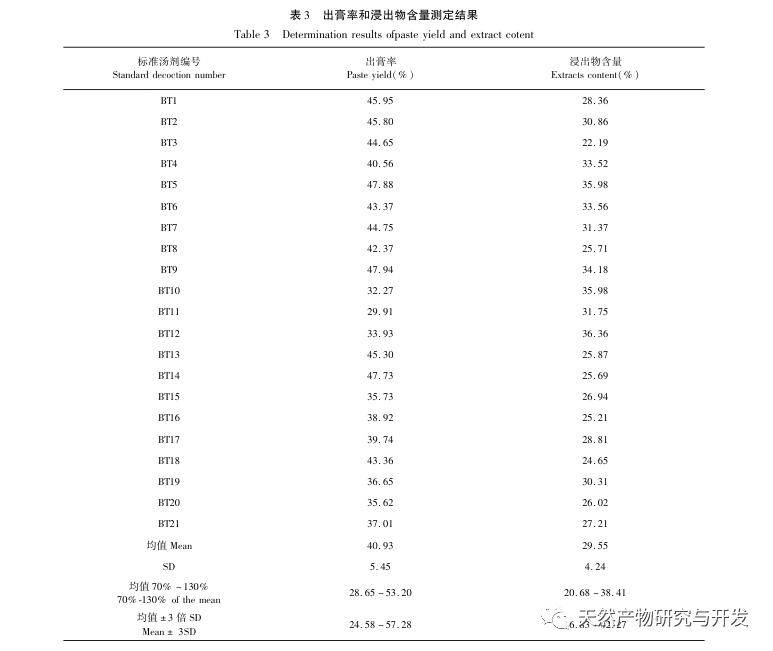
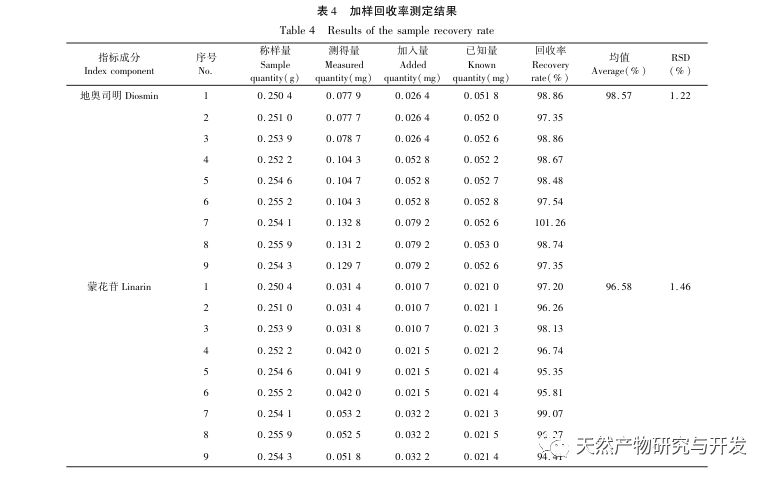
 Half lotus has a relatively light texture, and during the decoction process, a large amount of components overflow, which increases the difficulty of establishing fingerprint spectra. In the process of establishing fingerprint spectra, considering the information content, chromatographic peak response, and separation effect, three mobile phase systems were investigated: methanol-0.1% phosphoric acid solution, acetonitrile-0.1% phosphoric acid solution, and acetonitrile-0.1mol/L potassium dihydrogen phosphate solution. Finally, acetonitrile-0.1mol/L potassium dihydrogen phosphate solution was selected. By adopting a full wavelength scanning mode, the optimal absorption wavelength of the fingerprint spectrum was determined. The “total peak area of common peaks in the fingerprint spectrum/sample size” was used as the indicator to select the best preparation method for the test solution. The established fingerprint spectrum method was validated by systematic methodology and met the requirements. In the 2020 edition of the Chinese Pharmacopoeia, there are no content determination indicators under the category of “half lotus”. Referring to the “Hong Kong Chinese Herbal Medicine Standards”, Zeng and other research results, a method for determining the content of active ingredients diosmin and paeoniflorin has been established to guide the study of value transfer in standard decoctions. According to the investigation of the production area, half lotus is mainly produced in Guizhou, Anhui, Jiangxi, Hunan, Hubei, Henan and other places, with wild resources as the main source. To ensure the representativeness of the samples, our staff went deep into the main production areas of half lotus to collect samples to meet the needs of standard soup research. From the results of the extraction rate, extract, and content determination of the standard decoction, it can be seen that there are certain differences in the extraction rate, extract, and the content of diosmin and paeoniflorin in the 21 batches of Banbianlian standard decoction. The maximum extraction rate and extract are about 1.6 times the minimum value, and the maximum content of diosmin and paeoniflorin is 2-4 times the minimum value. This difference may be related to differences in the origin, harvesting time, and processing methods of medicinal herbs.
Half lotus has a relatively light texture, and during the decoction process, a large amount of components overflow, which increases the difficulty of establishing fingerprint spectra. In the process of establishing fingerprint spectra, considering the information content, chromatographic peak response, and separation effect, three mobile phase systems were investigated: methanol-0.1% phosphoric acid solution, acetonitrile-0.1% phosphoric acid solution, and acetonitrile-0.1mol/L potassium dihydrogen phosphate solution. Finally, acetonitrile-0.1mol/L potassium dihydrogen phosphate solution was selected. By adopting a full wavelength scanning mode, the optimal absorption wavelength of the fingerprint spectrum was determined. The “total peak area of common peaks in the fingerprint spectrum/sample size” was used as the indicator to select the best preparation method for the test solution. The established fingerprint spectrum method was validated by systematic methodology and met the requirements. In the 2020 edition of the Chinese Pharmacopoeia, there are no content determination indicators under the category of “half lotus”. Referring to the “Hong Kong Chinese Herbal Medicine Standards”, Zeng and other research results, a method for determining the content of active ingredients diosmin and paeoniflorin has been established to guide the study of value transfer in standard decoctions. According to the investigation of the production area, half lotus is mainly produced in Guizhou, Anhui, Jiangxi, Hunan, Hubei, Henan and other places, with wild resources as the main source. To ensure the representativeness of the samples, our staff went deep into the main production areas of half lotus to collect samples to meet the needs of standard soup research. From the results of the extraction rate, extract, and content determination of the standard decoction, it can be seen that there are certain differences in the extraction rate, extract, and the content of diosmin and paeoniflorin in the 21 batches of Banbianlian standard decoction. The maximum extraction rate and extract are about 1.6 times the minimum value, and the maximum content of diosmin and paeoniflorin is 2-4 times the minimum value. This difference may be related to differences in the origin, harvesting time, and processing methods of medicinal herbs.
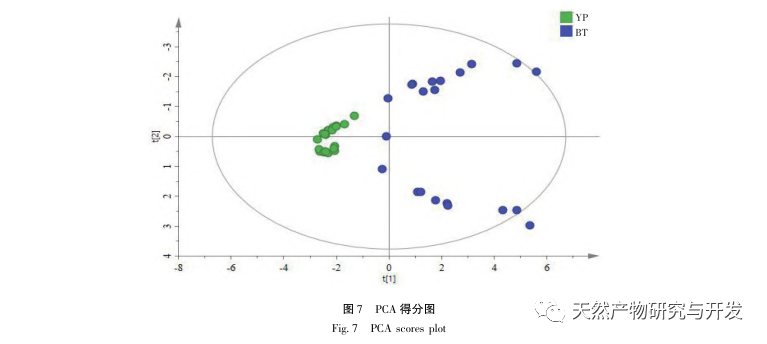
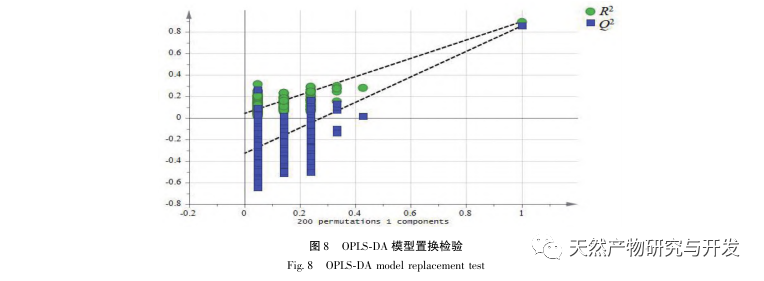
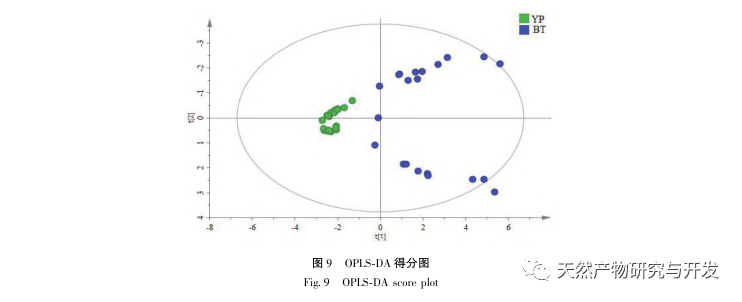
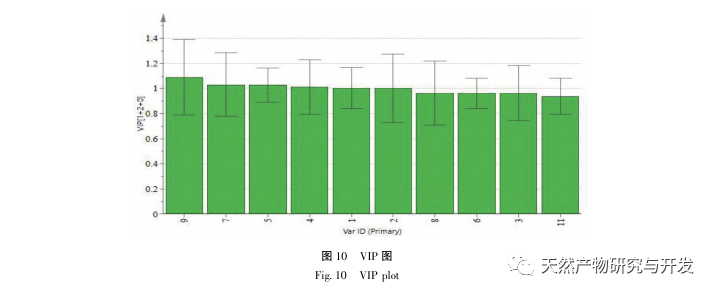
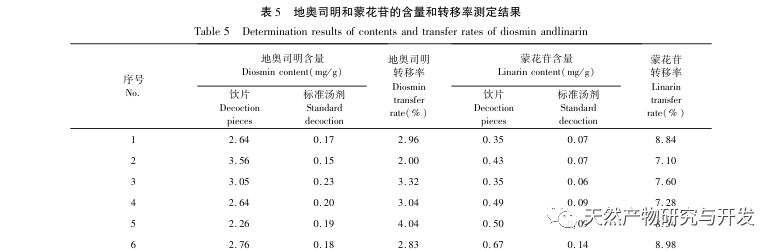
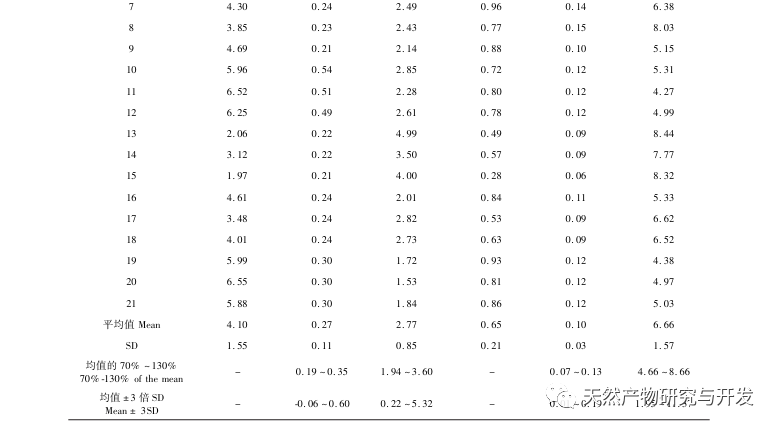
The 2020 edition of the Chinese Pharmacopoeia does not have any content determination indicators for the medicinal herb of Half Lotus. Flavonoids are a very important class of compounds in Half Lotus and are the main active ingredients for anti-cancer treatment. Among them, Diosmin is a flavonoid glycoside compound that has the effect of improving venous permeability and microcirculation. Montessorin and Diosmin have very similar structures and high content in Half Lotus. Therefore, Diosmin and Montessorin are used as content determination indicators for medicinal herbs and decoction pieces, which have certain specificity and practical significance. However, from the calculation results of the transfer rate, the transfer rates of diosmin and mogroside from decoction pieces to standard decoction are both less than 10%, indicating that their water solubility is not ideal. From the proportion of common peaks in the fingerprint spectrum, it can be clearly seen that the proportion of diosmin and mogroside chromatographic peaks in the total peak area of the fingerprint spectrum decreases significantly from decoction pieces to standard decoction. Considering the lack of legal reference standards with known concentrations for other components identified from the fingerprint spectrum of half lotus, further research is needed to analyze the transfer of water-soluble components. According to the PCA results, it can be seen that there is a significant change in the relative peak area of the common peaks from Banbianlian decoction to standard decoction. OPLS-DA identified five chromatographic peaks with significant changes in relative peak area, namely peak 9, peak 7, peak 5, peak 4, and peak 1, which are considered to be related to the differences in the transfer rates of the chemical components represented by each common peak.
In this study, the extraction rate, extract, fingerprint spectrum, content of active ingredients, and transfer rate of standard decoction were used as evaluation indicators to analyze the value transfer process from Banbianlian decoction pieces to standard decoction, providing reference for the development of quality standards for Banbianlian formula granules and related preparations.
