Study on the Improvement Effect and Mechanism of Ganoderma Polysaccharide on diabetes Nephropathy Mice
Diabetes nephropathy (DN) is one of the most common microvascular complications in diabetes patients. The damage to renal function caused by DN is one of the major causes of disability and death worldwide. Despite some progress in the treatment of DN in the past few decades, there are still a series of challenges. Traditional treatment methods often only slow down the progression of diseases by controlling blood sugar and blood pressure, without effectively curing or reversing their development. In addition, some patients may develop resistance or adverse reactions to current treatment methods, so there is an urgent need to find new treatment strategies.
Lingzhi is a traditional Chinese herb widely used in traditional Chinese medicine treatments in Asia and other regions. One of its main effective ingredients is Ganoderma lucidum polysaccharide (GLP). As a natural effective ingredient extracted from the traditional Chinese medicine Ganoderma lucidum, GLP is widely considered to be able to play a potential role in the treatment of diabetes and its complications due to its pharmacological activities such as enhancing immune function, anti-tumor, antioxidant, etc. Research has shown that GLP can exert its effects through various pathways, including regulating the immune system, anti-inflammatory, and antioxidant effects. These active ingredients have shown certain therapeutic potential in laboratory research and clinical practice, especially in the treatment of diabetes related diseases. These characteristics make GLP a promising natural drug for DN treatment.
In the development process of diabetic nephropathy, the expression changes of receptor for advanced glycation end products (RAGE), collagen type IV (COL-IV), and inducible nitric oxide synthase (iNOS) play a key role. RAGE, as a molecular mediator of various complications of diabetes, its activation can aggravate inflammatory reaction and oxidative stress, and promote kidney damage. COL-IV is an important component of the glomerular basement membrane, and its abnormal accumulation is associated with glomerulosclerosis. The overexpression of iNOS is closely related to renal inflammation and cell apoptosis. Therefore, detecting the expression of these proteins is crucial for understanding the pathological progression of DN and provides the possibility for exploring new therapeutic targets.
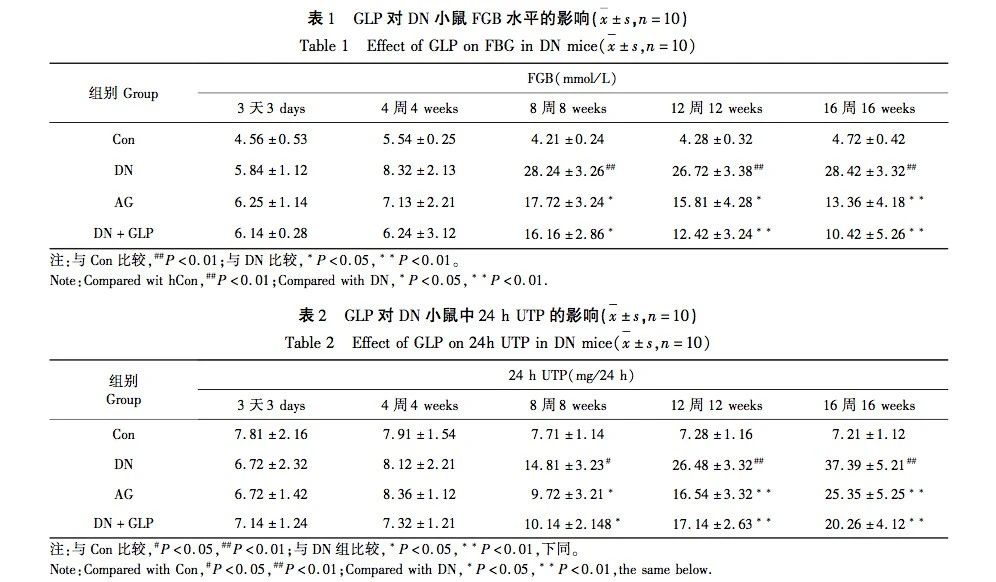
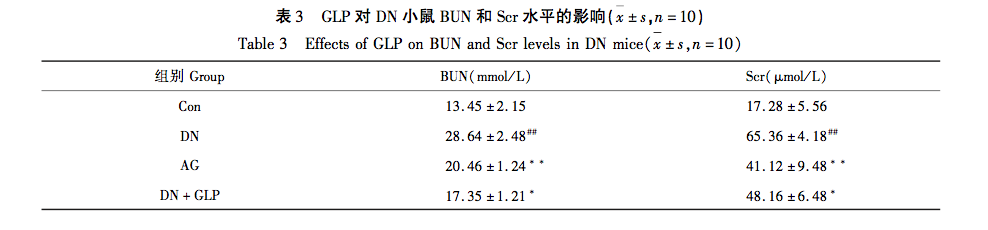




In order to evaluate the potential therapeutic effect of GLP on DN, a high-fat diet combined with streptozotocin (STZ) induced DN mouse model was used, and its mechanism of action was explored. Therefore, an aminoguanidine group was specially set as a positive control. By comparing the efficacy of GLP with aminoguanidine, the potential and effectiveness of GLP in treating DN can be preliminarily evaluated. Fasting blood glucose (FBG) is the blood glucose level measured in the fasting state after eating (usually 8-12h), which is usually used to assess the diagnosis and management of diabetes. 24-hour urine total protein (24-hour UTP) is the total amount of protein excreted through urine within 24 hours. When studying kidney diseases such as diabetic nephropathy, an increase in urinary glucose excretion may reflect damage to the glomerular filtration membrane. Blood urea nitrogen (BUN) is the concentration of urea nitrogen in the blood, and an increase in this level may indicate a decrease in renal filtration function, kidney disease, dehydration, high protein diet, and the presence of other factors. Serum creatinine (Scr), also known as serum creatinine, is commonly used as an indicator to evaluate glomerular filtration rate. Elevated levels of Scr can indicate decreased renal function. We will evaluate the pathological changes in the kidneys and the expression levels of related proteins by detecting FBG, 24-hour UTP, BUN, and Scr levels, as well as by histological and molecular biology methods. We speculate that GLP may improve the development of DN by inhibiting the RAGE signaling pathway. The development of this study will help fill the current knowledge gap in the treatment of DN and provide a theoretical and practical foundation for future clinical practice. At the same time, this study also provides a new direction and possibility for exploring a broader range of natural drugs to treat diabetes and its complications.
DN is one of the most common complications in diabetes patients, and its occurrence is mainly related to the damage of glomerulus and renal tubules caused by long-term hyperglycemia. During the development of DN, metabolic abnormalities and inflammatory reactions caused by diabetes lead to changes in the structure and function of kidney tissue, and ultimately lead to renal failure. Therefore, finding effective treatment methods is crucial for controlling the progression of diabetic nephropathy. GLP is a polysaccharide compound extracted from Ganoderma lucidum cells, which has various biological activities including antioxidant, anti-inflammatory, and anti-tumor effects. Recent studies have shown that GLP has certain potential in the treatment of diabetes and its complications, which may play a role by regulating inflammatory response, inhibiting oxidative stress and improving cellular signaling pathways. In this study, the AG group was specifically introduced as a positive control to enhance the scientific validity and persuasiveness of the research. As a widely recognized anti diabetes drug, AG has a clear scientific record of its therapeutic effect and mechanism on DN. By directly comparing the therapeutic effect of GLP with AG, not only can the efficacy benchmark of GLP be established, but also its mechanism of action in DN treatment can be deeply understood. In addition, the setting of the AG group also allows for the evaluation of the safety and tolerability of GLP, which is crucial for the clinical translation of any new drug. Through this comparison, the aim is to explore whether GLP can become a new and effective treatment option, or be used in combination with AG to improve treatment efficacy. Ultimately, these findings will provide valuable information for future research directions and may guide the optimization of DN treatment strategies in clinical practice.
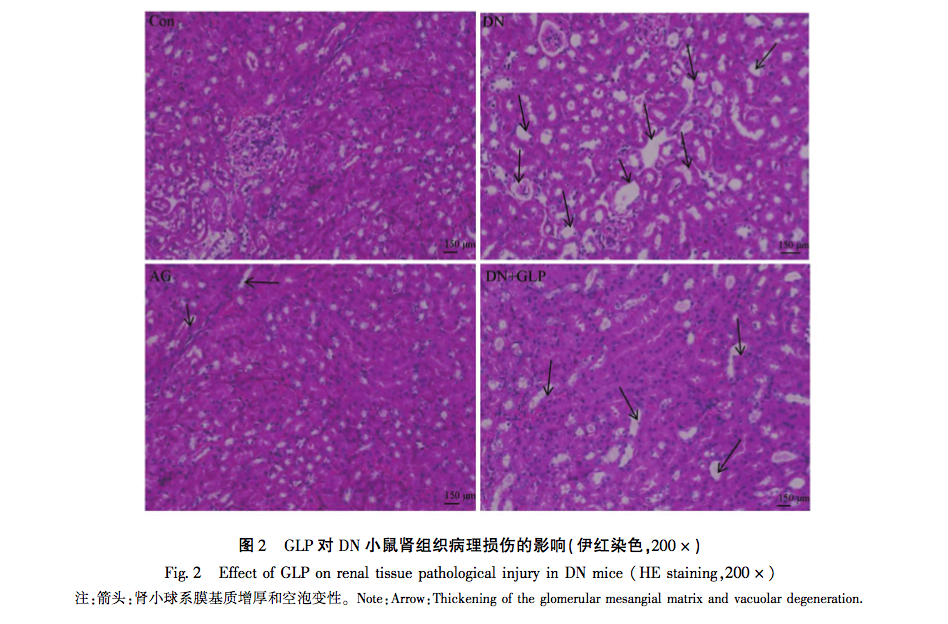
The aim of this study is to investigate the effects and potential mechanisms of GLP on DN mice induced by high-fat diet combined with STZ. Through a series of experiments, we found that compared with the control group, the levels of FGB, 24-hour UTP, BUN, and Scr in DN group mice were significantly increased, and the degree of renal pathological damage and fibrosis was significantly aggravated. At the same time, the levels of TNF – α and IL-6 in renal tissue also increased, while the level of anti-inflammatory factor IL-10 decreased. The renal pathological damage and fibrosis degree of AG group and DN+GLP group mice were significantly improved, and the relevant quantitative indicators were also significantly improved. In addition, significant increases in the expression of RAGE, COL-IV, and iNOS proteins were observed in DN mice, which is consistent with the pathological characteristics of DN and suggests that these proteins play important roles in the development of DN. After GLP intervention, it was found that the expression levels of these proteins were significantly downregulated, indicating that GLP may slow down the pathological progression of DN by inhibiting the expression of these key proteins. The downregulation of RAGE may reduce inflammation and oxidative stress, while the reduction of COL-IV may help alleviate glomerulosclerosis. Meanwhile, the decrease in iNOS expression may slow down the process of renal inflammation and cell apoptosis. These results suggest that the regulation of RAGE, COL-IV, and iNOS protein expression by GLP may be one of its key mechanisms for improving DN. The above results indicate that GLP has a certain degree of improvement effect on DN mice, and GLP may achieve its protective effect by inhibiting the RAGE signaling pathway. The experimental results show that GLP can significantly improve the degree of renal pathological damage and fibrosis in DN mice, reduce the biochemical indicators related to diabetes, and may play its protective role by inhibiting the RAGE signal pathway. Therefore, GLP may become a potential treatment for DN, and its mechanism may involve multiple biological effects such as anti-inflammatory, antioxidant, and anti fibrotic effects. Future research can further explore the regulatory mechanisms of GLP on the expression of these proteins and how to effectively intervene in DN through these pathways.
Of course, there are also some shortcomings in this study. Firstly, research can provide a deeper analysis of the mechanism by which GLP regulates inflammatory responses. Inflammatory response plays an important role in the development of DN, and GLP may alleviate renal inflammation by inhibiting the release of inflammatory mediators or regulating the activity of inflammatory signaling pathways, thereby protecting renal tissue from damage. Secondly, research can explore the regulatory mechanism of GLP on renal fibrosis. During the development of diabetic nephropathy, fibrosis of renal tissue is one of the main factors leading to renal dysfunction and disease progression. GLP may alleviate renal fibrosis by inhibiting collagen synthesis, regulating matrix metalloproteinase activity, or promoting collagen degradation, thereby protecting the integrity of renal structure and function. In addition, it is also important to explore the potential clinical application prospects of GLP in the treatment of DN. Although current research mainly focuses on animal models, the results provide strong support for GLP as a potential drug for treating DN. Further clinical studies will help determine the safety and efficacy of GLP in the treatment of human DN. Finally, researchers can compare the advantages and disadvantages of GLP and aminoguanidine in the treatment of diabetic nephropathy. Compared to traditional drugs, GLP may have a wider range of biological activities and fewer side effects. However, further research is needed to verify its long-term efficacy and safety, and to determine the optimal treatment plan and dosage.
