What are the proper labeling of food additives, net contents and specifications and labeling prohibitions?
Food net content and specifications labeling you have seen and read? Food net content and specification labeling seems to be a simple thing, but in daily life or from time to time will see some of the wrong labeling, I collected and organized some of the common problems for your reference.1 guide word errors
The title of the net content can only be labeled “net content” three words, labeling “net weight” “gross weight” “capacity” and so on belong to the net content title labeling. GB 7718-2011 National Standard for Food Safety General Principles for the Labeling of Prepackaged Foods 4.1.5.1 provides that: the labeling of net content should be composed of net content, numbers and legal units of measurement.
2 Unit Error
We often say “kilograms”, “pounds” and so on do not belong to the legal unit of measurement, and should use the legal unit of measurement “kilograms” or “kg should use the legal unit of measurement “kilogram” or “kg”, such as: “Net content: 1 kg”.
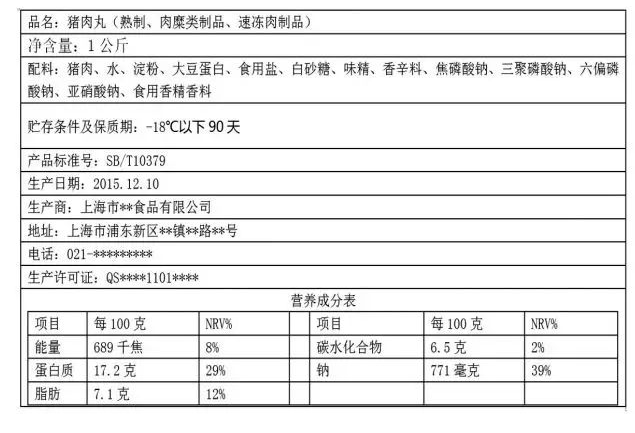
GB 7718-2011 General Rules for Labeling of Prepackaged Foods, National Standard for Food Safety 4.1.5.2 stipulates that: The net content of food in the package (container) shall be labeled according to the following forms based on the legal unit of measurement:
a) Liquid food, by volume liter (L) (l), milliliter (mL) (ml), or by mass gram (g), kilogram (kg); b) Solid food, by mass gram (g), kilogram (kg); c) Semi-solid or viscous food, by mass gram (g), kilogram (kg) or by volume liter (L) (l), milliliter (mL) (ml). The label does not use the unit of measurement (1000g) as required by the standard, which is not in line with the provisions of GB7718 General Principles for the Labeling of Prepackaged Foods 4.1.5.3. The net content of the label indicated value of 1000g, belongs to the net content of Q ≥ 1000g, the unit of measurement should be labeled as kilogram (kg), such as “net content / specifications: 1kg”.
GB7718 “General Principles for the Labeling of Prepackaged Foods” 4.1.5.3 of the provisions: the net content of the unit of measure of the labeling method, the following table:

Character height error
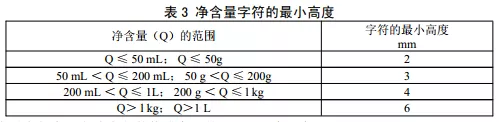
Some companies in the design and production of labels, often not “net content”, “digital characters” and “unit of measurement” as a whole, the emergence of only focusing on the height of the digital characters, while ignoring the “net content” and the height of the unit of measurement, resulting in the net content of the string as a whole does not meet the minimum height of the standard requirements. Net content” and the height of the unit of measurement, resulting in net content of the minimum height of the string as a whole does not meet the standard requirements. “Net content” includes three parts: net content, numbers and legal units of measurement. The height of all characters in the three parts should meet the requirements.
GB 7718-2011 “National Standard for Food Safety General Principles for Labeling of Prepackaged Foods” 4.1.5.4. The minimum height of the characters of net content should be in accordance with the provisions of Table 3.
4 Incorrect location of net content
Net content should be labeled on the same page with the name of the food so that consumers can easily see the labeling of net content while seeing the name of the food.
GB 7718-2011 National Standard for Food Safety General Principles for the Labeling of Prepackaged Foods 4.1.5.5 Net content should be labeled with the name of the food on the same display page of the package or container. Extended information “Food Safety National Standard FAQs”, page 91: 36. The product package is a cylinder, can the net content and the product name not be in the same visual layout of the package or container but in the same unfolding layout? No. The layout should be designed together and can be placed on the same visual layout when displayed. The net content should be labeled with the name of the food in the same layout in order to enable consumers to see the name of the food while easily seeing the labeling of the net content.
5 Unlabeled solids content
GB 7718-2011 “National Standard for Food Safety General Principles for the Labeling of Prepackaged Foods” 4.1.5.6 stipulates that when the container contains food with solid and liquid phase substances and the solid phase substances are the main food ingredients, in addition to the labeling of the net content, the content of leachate (solids) should also be labeled in the form of mass or mass fraction (see Appendix C for the labeling form).
Semi-solid, viscous food, solid-liquid phase are the main edible ingredients or suspended, solid-liquid mixture of prepackaged food can not clearly distinguish between solid-liquid phase products are not required to label the content of drained substances (solids). Prepackaged food due to its own characteristics, may be presented in different temperatures or other conditions in different forms of solid and liquid, is not solid and liquid two-phase food, such as honey, edible oils and other products.6 Combination and promotional packaging issues
GB 7718-2011 National Standard for Food Safety General Principles for the Labeling of Prepackaged Foods 4.1.5.7 When the same pre-package contains several single pieces of pre-packaged food, the large package should be labeled with specifications at the same time as the net content. The labeling of specifications should be composed of the net content of a single prepackaged food and the number of pieces, or only the number of pieces, may not be labeled with the word “specifications”. The specifications of a single piece of prepackaged food that refers to the net content, you can not additionally indicate the specifications.
The net content of prepackaged food for gift (or promotional) should be labeled, can be labeled as the net content of the promotional part of the net content and the net content of the gift part of the net content, you can also indicate the sales part of the total net content of the sales part and gift part and at the same time, with the appropriate way to indicate the net content of the gift part of the net content, such as “net content of 500 grams, 50 grams of gift”, “net content of 500 + 50 grams”, “net content of 500 + 50 grams”, “net content of 500 + 50 grams”, “net content of 500 + 50 grams”. “Net content of 500 + 50 grams”, ‘net content of 550 grams (including a gift of 50 grams)’ and so on.
Finally, when I collected information, I saw the following two labels, both net content labeling is feasible? Does it mean the same thing?
Food packaging, so that the identification of food additives is correct!
A lot of food in the production process, more or less will add food additives, then how to correctly identify food additives on the food label? Is every business must master, otherwise there will be business risks!
Today we summarize how to correctly identify food additives on food labels, I hope to help the majority of food companies, and welcome corrective exchanges.
The first point: food additives should be labeled with their common name of food additives in the “National Standard for Food Safety Food Additives Use Standard” (GB 2760-2014).
If two or more names are specified for a food additive in the Standard for the Use of Food Additives in National Standards for Food Safety (GB 2760-2014), each name is an equivalent common name. Take “sodium cyclohexylaminosulfonate (also known as sweetener)” as an example, “sodium cyclohexylaminosulfonate” and “sweetener” are both generic names.
The second point: the food additives used in the product should be truthfully labeled, but it is not mandatory to establish a “food additive item”. In the same prepackaged food labeling, the use of food additives and can choose to label in one of the following three forms:
1. All the specific names of food additives, the name of the food additives do not include its method of production, such as the production of ammonia, ordinary method, ammonium sulfite method of production of caramel color, in the label can be uniformly labeled as “caramel color”;
2. All food additives should be labeled with the names of their functional categories and international codes (INS numbers). If a certain food additive does not have a corresponding international code, or if the labeling of allergenic substances requires it, its specific name can be labeled, e.g., “Phospholipids” can be expressed as “Soybean Lecithin For example, “phospholipid” can be expressed as “soybean phospholipid”;
3. Label all the names of the functional categories of food additives and label the specific names at the same time. Food additives may have one or more functions, and the National Standard for Food Additives Use in Food Safety (GB 2760-2014) lists the main functions of food additives for reference. Production and management enterprises should follow the actual function of food additives in the product on the label to indicate the name of the functional category.
Example: The food additive “propylene glycol” can be optionally labeled as:
3.1 Propylene glycol;
3.2 Thickener (1520);
3.3 Thickener (propylene glycol).
When two or more food additives with the same function are added to the food, you may choose to label them with their respective specific names; or you may choose to label them with the name of the functional class first, followed by the specific name or international code (INS number) of the respective food additive.
Examples: “Carrageenan, Guar Gum”, “Thickener (Carrageenan, Guar Gum)” or “Thickener (407,412)”.
If a food additive does not have an INS number, it can be labeled with its specific name.
Examples: “Thickener (carrageenan, sodium polyacrylate)” or “Thickener (407, sodium polyacrylate)”.
Point 3: Labeling of Compounded Food Additives
Each food additive that has a functional role in the final product should be labeled in the food ingredient list. If the name of the compounded food additives is labeled, it should be noted that the naming rules of the compounded food additives should be in line with the provisions of the General Rules for Compounded Food Additives of National Food Safety Standards (GB 26687-2011). The name of the food additive should be “Compounding” + “Name of functional category of food additive in GB 2760” or “Compounding” + “Food category” + “GB 2760”. “ + “GB 2760 in the name of the functional category of food additives”, such as compounding moisture retention agent, or compounding meat products moisture retention agent. For example: a food additive compound coloring agent, can be marked as “compound coloring agent (natural carotene, amaranth red)” or in the ingredient list directly marked “natural carotene, amaranth red”.
Point 4: Labeling of Excipients in Food Additives
When the excipients contained in food additives do not play a functional role in the final product, they do not need to be labeled in the ingredient list. Excipients in food additives are food raw materials and food additives added for the purpose of processing, storage, standardization, dissolution and other processes of single or compounded food additives. These substances do not play a functional role in the food in which the food additive is used and do not need to be labeled in the ingredients again. For example, lutein containing edible vegetable oil, dextrin, antioxidants and other excipients can be directly labeled as “lutein”, or “coloring agent (lutein)” “coloring agent (161b)”. The coloring agent (161b)”.
Point 5: Labeling of enzyme preparations
If the enzyme preparation has lost its enzyme activity in the final product, it does not need to be labeled; if it still maintains its enzyme activity in the final product, it should be listed in the corresponding position of the ingredient list in accordance with the relevant provisions of the food ingredient list labeling according to the amount of enzyme added during the manufacturing or processing of the food.
For the processing aids, enzyme preparations and food additives that do not need to be labeled and do not play the role of auxiliary materials of the process, the enterprises can also be labeled in the ingredient list.
Point 6: Labeling of Food Nutritional Enrichment
Food nutritional fortification should be labeled in accordance with the “Standard for the Use of Food Nutritional Fortification” (GB 14880-2012) or the name announced by the former Commission on Health and Welfare.
Ingredients that can be used both as food additives or food nutritional fortifiers and as other ingredients should be labeled according to the standardization of the role they play in the final product. When used as a food additive, it should be labeled with the name specified in the National Food Safety Standards for the Use of Food Additives (GB 2760-2014); when used as a food nutrient enhancer, it should be labeled with the name specified in the Standard for the Use of Food Nutrient Enhancers (GB 14880-2012); when used as other ingredients, it should be labeled with its corresponding specific name. Name. For example. Monosodium glutamate (monosodium glutamate) can be used both as a condiment and as a food additive, when used as a food additive, it should be labeled as monosodium glutamate, when used as a condiment, it should be labeled as monosodium glutamate.
Summary of prohibitions on food labeling! (A) packaging design and materials: GB 4806.6-2016 National Standard for Food Safety Plastic Resins for Food Contact GB 4806.7-2016 National Standard for Food Safety Plastic Materials and Products for Food Contact GB 4806.8-2022 National Standard for Food Safety Paper and Cardboard Materials and Products for Food Contact GB 4806.10-2016 Food Safety National Standard Paint and Coating for Food Contact GB 4806.1-2016 National Standard for Food Safety General Safety Requirements for Food Contact Materials and Products GB 4806.9-2016 National Standard for Food Safety Metallic Materials and Products for Food Contact GB 4806.11-2016 National Standard for Food Safety Rubber Materials and Products for Food Contact GB 4806.10-2016 National Standard for Food Safety 2016 National Standard for Food Safety Paint and Coating for Food Contact GB 4806.5-2016 National Standard for Food Safety Glass Products GB 4806.4-2016 National Standard for Food Safety Ceramic Products GB 4806.3-2016 National Standard for Food Safety Enameled Products GB 4806.12-2022 National Standard for Food Safety Bamboo and Wood Materials and Products for Food Contact GB 13115-1991 Food Safety Standards Products GB 13115-1991 Food containers and packaging materials with unsaturated polyester resin and its glass fiber reinforced plastic products health standards GB 9685-2016 National standards for food safety Food contact materials and products with additives use standards GB/T 10004-2008 Packaging plastic composite film, bags dry composite, extrusion composite QB/T 1014-2010 Food packaging paper GB 23350-2021 Restriction of excessive packaging requirements for commodities Raw paper and materials for food and cosmetic packaging shall not use recycled waste paper, recycled plastics, phenolic resins, shall not use industrial-grade paraffin wax, food packaging ink, pigments shall not be printed in contact with food surface. Restrictions on excessive packaging requirements for commodities Food and Cosmetics requires that food shall not be over-packaged. For example, the packaging void ratio of primary processed food products shall not be greater than 10%, the packaging shall not exceed 3 layers, and the total cost of the outer packaging other than the initial packaging shall not be greater than 20% of the selling price of the food.
(ii) Not to display trademarks, names, packages and decorations identical or similar to those of well-known trademarks and well-known goods.
The Law of the People’s Republic of China Against Unfair Competition, the Trademark Law of the People’s Republic of China and the Regulations for the Implementation of the Trademark Law of the People’s Republic of China stipulate that: (i) no counterfeiting of another person’s registered trademark; (ii) no unauthorized use of trademarks, names, packages, decorations unique to well-known trademarks and well-known commodities or similar trademarks, names, packages, decorations on different or similar commodities; (iii) no use of identical or similar trademarks with other registered trademarks on the same or similar commodities. or similar goods; not to use trademarks identical with or similar to other registered trademarks on the same or similar goods; not to replace a registered trademark without the consent of the trademark registrant and put the goods of the replaced trademark back into the market (reverse counterfeiting); not to use marks identical with or similar to others’ registered trademarks as trade names or decorations on the same or similar goods, thus misleading the public; not to use “China Well-known Trademark” or “China Well-known Trademark” as a trade name or decoration on the same or similar goods without the consent of the trademark registrant. “China Well-known Trademark” shall not be used on commodities, commodity packages or containers, or used in advertisements, exhibitions and other commercial activities.
(iii) The labeling content shall not be ambiguous.
GB 7718-2011 National Standard for Food Safety General Principles for the Labeling of Prepackaged Food GB 28050-2011 National Standard for Food Safety General Principles for the Labeling of Prepackaged Food Nutrition Packaging, labeling, labeling content must be clear, eye-catching, durable, easy to recognize and read. Food or its packaging should be attached to the label, identification (laws and administrative regulations can not be attached to the food except). Should be directly marked in the smallest sales unit of food or its packaging, should be clear and conspicuous, the background and background color should be used to contrast, so that consumers are easy to identify, read.
(D) labeling content shall not have feudal superstitions, yellow content.
Packaging, labeling, labeling content must be easy to understand, accurate, scientifically based, shall not be marked with feudal superstitions, yellow, degrading other foods or contrary to scientific nutritional content. The content should be true and accurate, easy to understand, scientific and legal.
(E) labeling content shall not have the treatment of disease and other false, misleading, deceptive content.
General Principles for the Labeling of Prepackaged Foods”, ‘General Principles for the Labeling of Prepackaged Foods for Special Dietary Uses’, ‘The People’s Republic of China Anti-Unfair Competition Law’, ‘The People’s Republic of China Product Quality Law’, ‘The People’s Republic of China Protection of Consumer Rights and Interests Law’, ‘Food Labeling Regulations’, ‘Food Advertisement Regulations’ and other provisions: Packaging, labeling, labeling content shall not be false, mislead consumers or deceptive The contents of packaging, labeling and labeling shall not introduce food in a false, misleading or deceptive manner, nor shall they use font size or color difference to mislead consumers; they shall not use directly or indirectly suggestive language, graphics or symbols to cause consumers to confuse the purchased food or a certain nature of food with another product; all processed food shall not be added “fresh” or “fresh” on the packaging, labeling, labeling or in front of the name; the contents of packaging, labeling and labeling shall not be misleading. No “fresh” or “fresh” shall be added to the package, label, logo or before the name to indicate that the food is fresh and natural; the content shall be true and accurate, easy to understand, healthy and scientific, and shall not be false and misleading, and there shall be no medical terminology, publicity of therapeutic effects, terms that can be easily confused with medicines, and terms that can not be evaluated by objective indexes.
Article 73 of the Food Safety Law of the People’s Republic of China stipulates: “The contents of food advertisements shall be true and lawful, and shall not contain any false content, nor shall they involve disease prevention or therapeutic functions. It shall not forge or falsely label the production date and shelf life; it shall not forge the origin of food; it shall not forge or fraudulently use the name or address of other producers; it shall not forge, fraudulently use or alter the production license number, etc.. No counterfeiting, fraudulent use of product bar code; shall not be labeled with the following: (1) expressly or impliedly have the effect of preventing or treating diseases; (2) non-health food expressly or impliedly have the effect of health care; (3) deceptive or misleading way of describing or introducing the food; (4) additional product description can not be verified on the basis of the product; (5) the text or pattern does not respect the national custom, with discriminatory (6) the use of the national flag, national emblem or RMB for labeling;
Food packaging of the following terms are not allowed: 1. immune regulation; 2. regulation of blood lipids; 3. regulation of blood glucose; 4. delay aging; 5. improve memory; 6. improve vision; 7. promote lead; 8. clear throat; 9. regulate blood pressure; 10. improve sleep; 11. promote lactation; 12. anti-mutagenesis; 13. anti-fatigue; 14. hypoxia resistance; 15. anti-radiation; 16. weight loss; 17. Promote growth and development; 18. Improve osteoporosis; 19. Improve nutritional anemia; 20. Chemical liver injury has an auxiliary protective effect; 21. Cosmetology (acne / chloasma / improve skin moisture and oil); 22. Improvement of gastrointestinal function (regulating intestinal flora / promote digestion / laxative / gastric mucosa has an auxiliary protective effect); 23. Inhibition of tumors. May not be labeled as a disease prevention, mitigation, treatment or cure. May not be labeled “rejuvenation”, “prolonged life”, “gray hair into black”, “teeth fall more alive”, “anti-cancer” or other similar terms.
(vi) Absolutization and other terms shall not be used.
Article 7 of the Advertising Law of the People’s Republic of China stipulates: “The contents of advertisements shall be favorable to the physical and mental health of the people, promote the improvement of the quality of goods and services, and protect the legitimate interests of consumers. Terms such as national, supreme and best shall not be used in advertisements.” Prohibited words of advertising language that shall not be used: 1. National level, world level, highest level, best, first, only, first, best, precise, top, lowest, bottom, most, cheapest, maximum degree, latest technology, most advanced science, national level products, fill the gaps in the country, absolute, exclusive, first, newest, most advanced, first brand, gold medal, name brand, most profitable, super profitable, first, superstar, luxury, Supreme, top enjoyment and other absolute terms; 2, national XXX leaders recommended, national XX organs recommended, national XX organs exclusive, special supply and other terms borrowing the name of the country, the state organs staff to promote; 3, the quality of inspection-free, no need for national quality testing, free sampling, and other terms claiming that the quality does not need to be tested; 4, the use of Renminbi graphics (but except for those approved by the central bank); 5, traditional Chinese characters (trademarks) (except trademarks), the use of foreign words alone, or a combination of Chinese and English words; 6, ancestral, inhibition, secret system and other false words; 7, powerful, special effects, full effect, strong effect, miraculous effect, high efficiency, quick effect, miraculous effect and other words of exaggeration; 8, prescription, prescription, treatment, anti-inflammatory, anti-inflammatory, blood, blood circulation, cough, detoxification, efficacy, prevention and treatment, cancer prevention and treatment, cancer prevention and treatment, cancer resistance, tumors, height increase, puzzle, the name of a variety of diseases and other words that indicate or imply a therapeutic effect; 7, the use of the traditional Chinese characters (except for trademarks), the use of foreign words alone or the combination of Chinese and English words. Or imply that there is a therapeutic effect of the words; 9, the gods, gods and other vulgar or with feudal superstitious words.
(g) The content of the label must use Chinese characters (except for registered trademarks), and the use of pinyin, minority characters, foreign languages, which shall not be larger than the corresponding Chinese characters (except for registered trademarks).
Packaging, labeling, labeling content must use standardized Chinese characters (except for registered trademarks); can also use Pinyin or minority characters, but shall not be greater than the corresponding Chinese characters; can also use foreign languages, but should have a corresponding relationship with the Chinese characters (except for the manufacturer and address of imported food, the name and address of foreign distributors, and web site); all foreign languages shall not be greater than the corresponding Chinese characters (except for registered trademarks).
(H) mandatory labeling content shall not be less than 1.8 mm.
Packaging or packaging containers with a maximum surface area greater than 35cm2, the mandatory labeling content of the text, symbols, numbers, the height shall not be less than 1.8 mm. food or its packaging is less than the maximum surface area of 10cm2, you can only label the name of the food, the producer’s name and address, the net content and date of production and shelf life. Food name, list of ingredients, net content, factory name and address, date of production, shelf life, product standard number for the standard mandatory labeling content. If through the outer packaging can clearly identify the inner packaging or container on all or part of the mandatory labeling content, you can not repeat the labeling of the corresponding content on the outer packaging; if the inner packaging (or container) outside another direct-to-consumer delivery of the outer packaging (or packaging), you can only mark the outer packaging (or packaging) on the mandatory labeling content.
(Ix) food labeling, marking shall not be separated from the packaging (container).
Labeling, marking shall not be separated from the food or its packaging (container).
(J) food name labeling must be conspicuous, prominent, indicating the true nature of the food, shall not shrink, obscure, ambiguous.
General Principles for the Labeling of Prepackaged Foods”, ‘General Principles for the Labeling of Prepackaged Foods for Special Dietary Uses’, ‘Food Labeling Regulations’:
Packaging, labeling, marking should be in a conspicuous position, clearly marked to indicate and reflect the real attributes of the food special name;
Foods made from animal or plant food as raw materials and using specific processing techniques to imitate the characteristics of individuals, organs and tissues of other organisms should be labeled with the words “artificial”, “imitation” or “vegetarian” before the name. The name should be preceded by the words “artificial”, “imitation” or “vegetarian” and labeled with the name of the classification (genus) of the real attributes of the food;
When one or several names of a food have been stipulated in the national or industrial standards, one of them, or an equivalent name, shall be used;
When there is no name stipulated in the national standards or industry standards, the commonly used name or common name should be used so as not to mislead or confuse the consumers;
Can be labeled “new name”, “peculiar name”, “phonetic name”, “brand name”, “regional slang name” or “trademark name‘’, but the name shall be labeled adjacent to the name shown with any one of one or more names or equivalent names of the food that have been stipulated in national standards or industry standards. (b) The name of the food shall not be used as a substitute for the name of the food;
(b) When the name is a “newly created name”, a “peculiar name”, a “phonetic name”, a “brand name”, or a “regional slang name”, “regional slang name” or ‘trademark name’ contains words or terms (phrases) that can easily mislead people into misunderstanding the attributes of the food, the same font size should be used in the neighboring part of the name shown to indicate the exclusive name of the real attributes of the food;
When the special name of the real attributes of the food is easy to mislead people to understand the attributes of the food due to different font sizes, the same font size should also be used to indicate the special name of the real attributes of the food. For example, “Orange Juice” and “Drink” in “Orange Juice Drink”, “Chocolate” and “Chocolate” in “Chocolate Sandwich Cookies”, and “Chocolate” and “Chocolate” in “Chocolate Sandwich Cookies”. “chocolate”, ‘sandwich cookies’, should use the same font size; to avoid consumer misunderstanding or confusion about the real properties of food, physical state or production methods, can be attached to the name of the food before or after the name of the food or the corresponding words or phrases. Such as dried, concentrated, restored, smoked, fried, powdered, granular.
The name of a food consisting of two or more foods that are physically mixed and have a uniform and consistent appearance that is difficult to separate from each other shall reflect the mixed properties and the classification (genus) name of the food;
Before and after the name of the food, shall not be crowned with the name of the drug or with the drug graphics, names (excluding substances used for both medicinal and dietary purposes) implying therapeutic effects, health care functions.
In order to meet the physiological needs of certain special groups of people, or the nutritional needs of patients with certain diseases, food (including baby food) specially processed according to a special formula, i.e., food for special dietary use, can be used in the name of such as “infant formula milk (milk) powder”, “sugar-free instant soybean powder” (for diabetic patients). “(for diabetic patients), “fortified with iron and high protein instant soybean meal” (for patients with anemia) and other modifiers of special significance.
(XI) labeling content must have a list of ingredients.
Prepackaged food should be labeled with a list of ingredients. List of ingredients should be “ingredients” or “ingredient list” as the title; a variety of ingredients should be added according to the manufacturing or processing of food in decreasing order one by one, the addition of no more than 2% of the ingredients can not be arranged in decreasing order; in the food directly using sweeteners, Preservatives, coloring agents in the food directly used, should be marked in the list of ingredients under the food additives specific name; the use of other food additives, you can mark the specific name, type or code.
(XII) net content labeling shall not use non-statutory units of measurement, and should be displayed with the name of the food in the same layout.
Quantitative prepackaged food should be labeled net content, net content should be labeled by the net content, numbers and legal units of measurement. Such as “net content of 450g”, or “net content of 450 grams”;
Should be based on the “supervision and management of quantitative measurement of packaged goods,” the legal unit of measurement, according to the following ways to indicate the net content of food in the packaging (containers): 1, liquid food, with the volume – L (l) (l) (mL) (ml) (milliliters); 2, solid food, with the mass of – g (grams), kg (kilograms); 3, semisolid or viscous foods, use mass or volume. The net content should appear on the same display page of the wrapper or container as the name of the food. Foods containing both solid and liquid phase substances in the container (e.g. canned pears in sugar water) should be labeled with the content of drained substances (solids) in addition to the net content. Expressed as mass or mass fraction.
Example: Net content of canned pears in sweetened water: 425 g drained (also labeled as solids or pear pieces), not less than 255 g (or not less than 60%)
The same prepackaged if they contain several independent of each other the same prepackaged food, in the labeling of net content at the same time should also indicate the number of food or the number of pieces, excluding large packages of non-single sales of small packages, such as small pieces of candy.
(M) must be labeled factory name and address.
Prepackaged food should be labeled food food producer’s name, address and contact information. Should be labeled food origin, food origin should be labeled in accordance with administrative divisions to the prefecture level territory. Producer’s name and address should be registered according to law, can assume responsibility for product safety and quality of the producer’s name and address. One of the following circumstances shall be labeled in accordance with the following provisions:
1. according to law independently assume legal responsibility for the group, the group’s branches (subsidiaries), should be labeled with their respective names and addresses.
2 according to law can not independently assume legal responsibility for the group’s branches (subsidiaries) or the group’s production base, you can label the group and branch (production base) of the name and address, you can also only label the name and address of the group company.
3. commissioned by other units to process prepackaged food but does not undertake external sales, should be marked with the name and address of the commissioning unit; for the implementation of the production license management of food, commissioned by the enterprise with its commissioned processing of food production license should be marked with the name of the commissioning enterprise, address and the name of the commissioned enterprise, or only the name and address of the commissioning enterprise.
4. Imported prepackaged food should be labeled with the name of the country of origin or regional name (referring to Hong Kong, Macao, Taiwan), as well as registered in China according to law, the agent, importer or distributor’s name and address.
5. Food should be labeled with the name and address of the sub-packaging, and indicate the word sub-packaging.
(XIV) must be labeled production date, shelf life, storage conditions.
The date shall not be additionally affixed, supplemental printing or tampering.
(XV) labeling content must have product standard number.
Domestic production and domestic sales of packaged food (excluding imported prepackaged food) should be labeled with the implementation of national standards, industry standards, local standards, or by the filing of the enterprise standard code and sequence number.
(P) must be labeled quality (quality) level (product standards are divided into quality (quality) level).
Enterprise implementation of product standards have been clearly defined quality (quality) level of products, should be labeled quality (quality) level, processing technology.
(XVII) must be marked with a food production license number (food production license system into the management of food).
The implementation of production license management of food, food production license number should be marked. Commissioned the implementation of production and processing of food production license management, commissioned by the enterprise has its commissioned the processing of food production license can be marked commissioned by the enterprise or commissioned by the enterprise’s production license number.
(XVIII) mixed non-food products can easily cause accidental injury to the person, shall be marked with a warning sign or Chinese warning instructions.
Mixed non-food products easy to cause accidental consumption, improper use, easy to cause personal injury, should be marked on its logo warning signs or Chinese warning instructions.
(XIX) alcohol content greater than 0.5% of the alcohol and must be labeled health warnings; glass bottles of beer must be labeled safety warnings.
(XX) marked with “nutrition”, “fortified” words, to label the food nutrients and calories.
Food in its name or description of the label “nutrition”, “fortified” words, should be in accordance with relevant provisions of national standards, labeling the food nutrients and calories, and in line with national standards for quantitative labeling.
(XXI) Other mandatory labeling content
Irradiated food: food treated with ionizing radiation or ionizing energy shall be marked with “irradiated food” near the name of the food; any ingredient treated with ionizing radiation or ionizing energy shall be marked in the list of ingredients.
Genetically modified foods or foods containing legal genetically modified raw materials: they should be labeled with GMO labels such as “genetically modified XX food” or “genetically modified XX food as raw material”.
Special Foods: Labeling of suitable people and warning words are required.
For example, beverages containing caffeine, vitamin B6, vitamin B12 and other ingredients must be labeled with the maximum daily limit. 2. Low-fat milk, skimmed milk, skimmed milk powder, dairy beverages and other food products should be labeled with the words “can not be a complete substitute for baby food” and “skimmed milk is not suitable for, or can not be used as, baby food”. 3. Foods containing royal jelly should be labeled “may cause a variety of allergic reactions, especially for people with asthma and a history of allergies may be fatal”. 4. Caffeine-added beverages, in addition to the caffeine content must be labeled, should also be labeled “not for children, Caffeine-added beverages, in addition to the caffeine content, should be labeled as “not suitable for children, pregnant women, lactating mothers and those allergic to caffeine”.
Sugar-free food: The General Principles for the Labeling of Prepackaged Foods for Special Dietary Uses clearly stipulates that the requirement of “sugar-free” refers to the sugar content of solid or liquid foods of not more than 0.5 grams per 100 grams or 100 milliliters. If a product is labeled as “sugar-free”, its content of disaccharide (such as sucrose, lactose, maltose, etc.) or monosaccharide (such as glucose, fructose, etc.) must meet the above standard.
(xxii) Genetically modified food:
Genetically modified food, refers to the use of genetic engineering techniques to change the genome composition of animals, plants and micro-organisms produced food and food additives, including: genetically modified animals, plants and micro-organisms products; genetically modified animals, plants and micro-organisms directly processed products; genetically modified animals, plants, micro-organisms, or their directly processed products as raw materials for the production of food and food additives.
(1) Genetically modified food can be produced or imported only after it has been examined and approved by the Ministry of Health and labeled with an approval number. The Food Safety Law of the People’s Republic of China and the Measures for the Administration of New Resource Foods stipulate that genetically modified food, as a kind of new resource food, shall be examined and approved by the Ministry of Health and labeled with an approval number before it can be produced, sold or imported. The approval numbers are “卫新食试字”, “卫新食准字”, “卫新食進试字”, “卫新食进准字”, “卫新食进准字 “ + “(××××)” year + “No. x x”. Genetically modified foods that have not been examined and approved by the Ministry of Health may not be produced for sale or imported, nor may they be used as food or food ingredients. Approved genetically modified foods shall be listed by the Ministry of Health in the catalog of genetically modified food varieties that can be used for food production and operation. The name of the genetically modified food shall be consistent with the content approved by the Ministry of Health.
(2) Genetically modified foods must be labeled with an agricultural genetically modified organism label. Regulations on the Safety Management of Agricultural Genetically Modified Organisms, Law of the People’s Republic of China on Quality and Safety of Agricultural Products, Measures for the Administration of New Resource Foods, Food Safety Law of the People’s Republic of China, Measures for the Administration of Labeling of Genetically Modified Organisms, Measures for the Administration of Packaging and Labeling of Agricultural Products, etc.: the state shall implement a labeling system for agricultural GMOs, and agricultural products included in the catalog of agricultural GMOs shall be Agricultural products included in the list of agricultural GMOs should be labeled; those that are not labeled or are not labeled in accordance with the regulations shall not be imported or sold. Agricultural genetically modified organisms included in the agricultural genetically modified organisms catalog shall be labeled by production and distribution units and individuals; if they are not labeled, they shall not be sold. Operating units and individuals shall check the goods and labeling when they purchase goods. Operating units and individuals who disassemble the original packaging for sale shall be re-labeled. Food products (including raw materials and processed food) containing genetically modified organisms or/and expression products shall be labeled as “genetically modified XX food” or “genetically modified XX food as raw material”. If the genetically modified food comes from potentially allergenic food, it should also be labeled as “This product has been genetically modified to XX food, attention to people with XX food allergy”. Genetically modified food shall be labeled in the following ways: (1) in the case of shaped package, in a conspicuous position on the label; (2) in the case of bulk package, in the price tag or on a notice board set up separately; (3) in the case of transshipment, in the delivery note; (4) in the case of importation, in the trade contract and the customs declaration.
Labeling methods: (1) genetically modified plants and animals (including seeds, breeding livestock and poultry, aquatic fry) and microorganisms, genetically modified plants and animals, microbial products, containing genetically modified plants and animals, microorganisms or their products, seeds, breeding livestock and poultry, aquatic fry, pesticides, veterinary drugs, fertilizers and additives, and other products, and directly labeled with “genetically modified × × ×”. “. (2) genetically modified agricultural products directly processed products, labeled as “genetically modified × × processed products (manufactured products)” or “processing raw materials for genetically modified × ×”. (3) Agricultural genetically modified organisms or products processed with products containing agricultural genetically modified organisms, but the final sale of the product no longer contains or can not detect genetically modified ingredients in the product, labeled as “This product is genetically modified × × processed products, but this product no longer contains genetically modified ingredients” or labeled as “This product has GM x x in the processing raw materials, but this product no longer contains GM ingredients”.
The first batch of agricultural genetically modified organisms directory for the implementation of labeling management i. Soybean seeds, soybeans, soybean meal, soybean oil, soybean meal ii. Corn seeds, corn, corn oil, corn meal (including corn meal with tax codes 11022000, 11031300, 11042300) iii. Rapeseed, rapeseed, rapeseed oil, rapeseed meal iv. Cotton seeds v. Tomato seeds, fresh tomato, Tomato paste
Genetically modified organisms labeling methods are three: First, genetically modified plants and animals (including seeds, breeding livestock and poultry, aquatic fry) and microbial products, containing genetically modified plants and animals, microorganisms or their product components such as seeds, breeding livestock and poultry, aquatic fry, pesticides, veterinary drugs, fertilizers and additives, and other products, directly labeled as “genetically modified x × x”.
Second, the direct processing of genetically modified agricultural products, labeled as “genetically modified × × processed products (manufactured products)” or “processing raw materials for genetically modified × ×”.
Third, the use of agricultural genetically modified organisms or products made from products containing agricultural genetically modified organisms, but the final sale of the product no longer contains or can not detect genetically modified components of the product, labeled as “this product is genetically modified × × processed products, but this product no longer contains genetically modified components”, or labeled as “The raw materials of this product are genetically modified ××, but this product no longer contains genetically modified ingredients”.
The Ministry of Agriculture is responsible for the validation and supervision and management of the labeling of agricultural genetically modified organisms throughout the country, and the labeling of imported agricultural genetically modified organisms can only be used after being examined and approved by the Ministry of Agriculture, and at the same time copied to the General Administration of Quality Supervision, Inspection and Quarantine of the State, the Ministry of Foreign Trade and Economic Cooperation, etc.; the labeling of domestic agricultural genetically modified organisms can only be used after being examined and approved by the competent department of agriculture administration of local people’s governments at or above the county level, where the production and dispensing units of the agricultural genetically modified organisms and individuals are located can only be used after the provincial administrative department of agriculture uniformly reported to the Ministry of Agriculture for the record. The State Administration of Quality Supervision, Inspection and Quarantine (AQSIQ) is responsible for the labeling inspection and verification of imported agricultural GMOs at the ports of entry.
(3) The labeling of agricultural genetically modified organisms shall be conspicuous and shall be designed and printed at the same time as the packaging and labeling of the products, or shall be separately labeled and described.
Regulations on the Safe Management of Agricultural Genetically Modified Organisms, Measures for the Administration of Labeling of Genetically Modified Organisms: The labeling of agricultural genetically modified organisms shall be conspicuous and designed and printed at the same time as the packaging and labeling of the products. If it is difficult to label agricultural genetically modified organisms on the original package or label, the original package or label can be labeled with additional genetically modified organisms, but the additional labeling should be firm and long-lasting. When it is difficult to label agricultural genetically modified organisms with packages or labels, the following methods of labeling may be adopted:
(1) Agricultural genetically modified organisms in fast food and retail trade that are difficult to label on each product for sale may be labeled on the product display (show) cabinet (table), or on the price tag or set up a labeling board (plate) for labeling.
(2) The sale of agricultural genetically modified organisms without packaging and labeling can be marked by setting up identification boards (plates).
(3) When agricultural genetically modified organisms in transportation containers are sold directly without packaging, the sales site can mark the containers or set up identification boards (plates) for identification.
(4) When selling unpackaged and unlabeled agricultural genetically modified organisms and it is difficult to mark them with identification boards (plates), the seller shall make a statement in an appropriate manner.
(5) imported unpackaged and unlabeled agricultural genetically modified organisms, it is difficult to label with identification plates (cards), should be indicated in the declaration of inspection (customs).
(4) agricultural genetically modified organisms labeling should use standardized Chinese characters for labeling. Agricultural genetically modified organisms labeling should use standardized Chinese characters for labeling. There are special sales requirements of agricultural genetically modified organisms, but also should be clearly marked the scope of sales, can be labeled as “limited to × × sales (production, processing, use).
(5) The labeling of genetically modified food shall not contain false content such as treatment of diseases. The labeling of genetically modified food shall be true and objective, and shall not expressly or impliedly cure diseases; shall not falsely and exaggeratedly publicize the role of the product; and shall not claim or imply that it has therapeutic effects and specific health functions.
(XXIII) imported food:
1, there must be Chinese labels, instructions. Labeling, instructions in line with the above general requirements used in food. Should also indicate the country of origin and the name of the agent within the name, address, contact information. 2, since July 28, 2015 after the import of food need to see the “Certificate of Entry Inspection and Quarantine of Goods”, proof that the Remarks column is marked with the name of the goods, the brand, the country of origin (region), specifications, the number / weight, date of production and other details.
