On March 13, 2024, the National Health Commission issued an announcement on 23 “Three New Foods” including Dendrobium procumbens, approving Dendrobium procumbens, (3R,3’S)-Dihydroxy-beta-carotene (endoxylated zeaxanthin), Clovis Picrytis, Bacillus subtilis DE111, L-alpha-glycophosphatidylcholine, and Streptomyces pseudoentericus as new food ingredients. and Pseudoenteric Membrane Bright Streptomyces as new food ingredients.
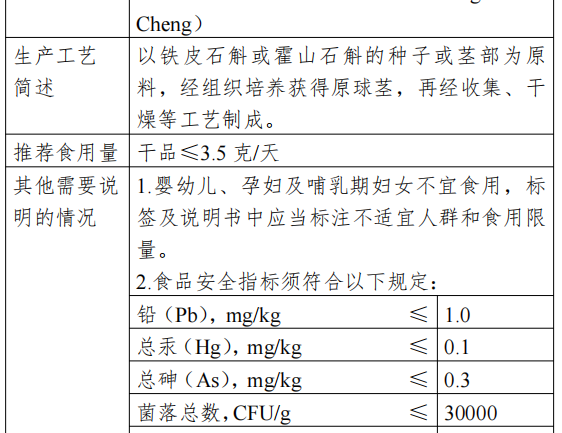
Dendrobium officinale (Latin name: Dendrobium officinale Kimuraet Migo) and Dendrobium huoshanense (Latin name: Dendrobium huoshanense C.Z. Tang et S. J. Cheng) are plants of Dendrobium genus of the Orchidaceae family, which have a history of food consumption in China’s folklore, and the main ways of food consumption are ready-to-eat, soup, cooking, stir-frying, juicing and brewing. The main ways of consumption are ready-to-eat, soup, cooking, stir-fry, juice, brewing and so on.
Dendrobium bulb is made from the seeds or stems of Dendrobium or Dendrobium Huoshan, which is cultured to obtain the bulb and then collected and dried. It contains dietary fiber, protein, dendrobium polysaccharide and other nutrients.
According to the “Food Safety Law of the People’s Republic of China” and “Administrative Measures for the Review of the Safety of New Food Ingredients”, the National Health and Health Commission commissioned the review body in accordance with the statutory procedures, and organized experts to review and pass the safety assessment materials of Dendrobium protocorm.
The production and use of new food ingredients should be in line with the content of the announcement and the requirements of food safety-related regulations. In view of the insufficient information on the safety of the consumption of Dendrobium bulb in infants and young children, pregnant women and lactating women, from the principle of risk prevention, the above groups of people should not be consumed, and the label and instructions should be labeled with the inappropriate groups and consumption limits. The food safety index of this raw material is in accordance with the announcement.
(3R,3’S)-Dihydroxy-beta-carotene
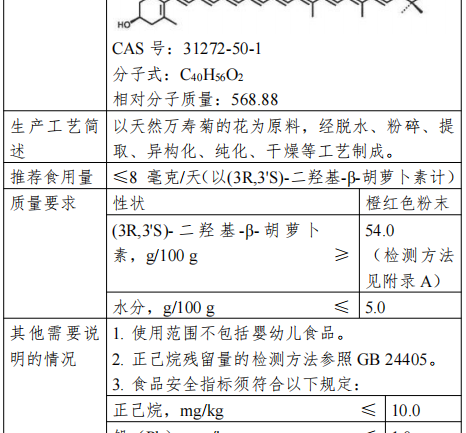
(3R,3’S)-Dihydroxy-beta-carotene, also known as endoxylated zeaxanthin, is made from the flowers of natural marigolds (Tagetes erecta L.) through dehydration, pulverization, extraction, isomerization, purification, and drying.
(3R,3’S)-Dihydroxy-beta-carotene is regulated as a Generally Recognized As Safe (GRAS) substance in the United States, and can be used in baked goods, beverages, dairy products and other foods, with a recommended use level of 0.18-1.8 mg/serving (in terms of (3R,3’S)-Dihydroxy-beta-carotene); It is used as a natural health food ingredient in Canada.
The recommended serving size of this product is ≤ 8 mg/day (as (3R,3’S)-dihydroxy-beta-carotene), and the content of 54.0-100.0 g/100 g is converted according to the actual content. The scope of use of (3R,3’S)-dihydroxy-beta-carotene does not include foods for infants and young children.
According to the “Food Safety Law of the People’s Republic of China” and “Administrative Measures for the Review of the Safety of New Food Ingredients”, the National Health and Health Commission commissioned the review body in accordance with the statutory procedures, and organized experts to review the safety assessment materials of (3R,3’S)-dihydroxy-beta-carotene and passed.
The production and use of the new food ingredient shall be in accordance with the content of the announcement and the requirements of food safety related regulations. The food safety index of this ingredient is in accordance with the announcement.
Kluwer Picchu yeast

Pichia kluyveri belongs to the Pichia genus Kluyveri, which is isolated from the grape fermentation process.
Pichia kluyveri is regulated as “Generally Recognized As Safe (GRAS)” in the U.S.A., and can be used as a fermentation strain for the fermentation of beer, fruit and vegetable juices, and teas, etc.; this strain has been listed in the Bulletin of the IDF 514/2022, “Proven in Fermented Foods”. “Catalog of Microbial Species Demonstrated to be Safe in Fermented Foods” and in the Danish “Record of the List of Microbial Strains Used in Food”.
This approval is included in the “List of Strains that can be Used in Foods”, and the scope of use includes fermentation and processing of fermented wines, fruit and vegetable juices and beverages, tea beverages, protein beverages, and botanical beverages, excluding foods for infants and young children.
According to the “Food Safety Law of the People’s Republic of China” and “Administrative Measures for the Review of the Safety of New Food Ingredients”, the National Health and Health Commission commissioned the review body in accordance with the statutory procedures, and organized experts to review the safety assessment materials of Kluwer’s Picchu yeast and passed.
The production and use of new food ingredients shall be in accordance with the contents of the announcement and the requirements of food safety related regulations. The food safety indexes of this raw material shall comply with the provisions of the National Standard for Food Safety Strains Preparations for Food Processing (GB 31639-2023).
Bacillus subtilis DE111

Bacillus subtilis DE111 is a Bacillus species of the genus Bacillus, isolated from traditionally fermented dairy products.
Bacillus subtilis has been included in the list of recommended biologics in the QPS list of the European Food Safety Authority (EFSA) as well as in the “List of microbial species proved to be safe in fermented foods” in the Bulletin of the IDF 514/2022, and is approved for use in many countries and regions, such as Japan and South Korea. It has been approved for use in many countries and regions, including Japan and Korea. Bacillus subtilis DE111 is used as a natural health food ingredient in Canada and as a health-related food in Australia.
According to the Food Safety Law of the People’s Republic of China and the Administrative Measures for the Review of the Safety of New Food Ingredients, the National Health and Wellness Commission has commissioned a review body to review the safety assessment materials of Bacillus subtilis DE111 in accordance with the statutory procedures, and organized experts to review and pass the materials, and the scope of use does not include food for infants and young children.
The production and use of new food ingredients should be in line with the content of the announcement and the requirements of food safety related regulations. The food safety indexes of this raw material shall comply with the provisions of the National Standard for Food Safety Strains Preparations for Food Processing (GB 31639-2023).
L-alpha-Glyphosphorylcholine
Choline L-alpha-glycophosphate is made from polyphosphoric acid, choline chloride, R-3-chloro-1,2-propanediol, sodium hydroxide, and water by condensation and esterification, followed by decolorization, decontamination, concentration, refining, and drying.
L-α-Choline Glycophosphate is regulated as a “Generally Recognized As Safe (GRAS)” substance in the United States, and L-α-Choline Glycophosphate is approved for use as a natural health product in Canada. The recommended serving size for this product is ≤600 mg/day (on a dry basis).
According to the Food Safety Law of the People’s Republic of China and the Administrative Measures for the Review of the Safety of New Food Ingredients, the National Health and Wellness Commission has commissioned the review body to organize experts to review and pass the safety assessment materials of L-α-glycylphosphatidylcholine in accordance with the statutory procedures.
The production and use of new food ingredients should be in line with the content of the announcement and the requirements of food safety-related regulations. In view of the insufficient information on the safety of consumption of L-α-glycylphosphorylcholine in infants and young children, pregnant women and lactating women, from the principle of risk prevention, the above groups of people should not be consumed, and the labeling and instructions should be marked as inappropriate for the population. The food safety index of this raw material is in accordance with the announcement.
Streptomyces pseudomallei
Leuconostoc pseudomesenteroides belongs to the genus Leuconostoc and is isolated from traditional fermented dairy products. This strain has been included in the list of recommended biologics in the QPS list of the European Food Safety Authority (EFSA) as well as in the Bulletin of the IDF 514/2022 “Catalogue of microbial species proved to be safe in fermented foods”, and has been approved for use in Denmark, Canada, Korea and other countries. Denmark, Canada, Korea and other countries have been approved for use.
The NHSC Announcement No.1 of 2023 approved the inclusion of this strain in the “List of Strains that can be Used in Foods”, and the scope of use includes fermented milk, flavored fermented milk, cheese, fermented milk beverages, and lactobacillus beverages (non-solid beverages), excluding foods for infants and young children. This application expands the scope of use to thin cream, cream (butter) and anhydrous cream (anhydrous butter).
According to the “Food Safety Law of the People’s Republic of China” and “Administrative Measures for the Review of the Safety of New Food Ingredients”, the National Health and Health Commission commissioned the review body in accordance with the statutory procedures, and organized experts to review and pass the safety assessment materials of Pseudointestinal membrane Ming Streptomyces.
The production and use of new food ingredients shall be in accordance with the contents of the announcement and the requirements of food safety related regulations. The food safety indicators of this raw material shall comply with the provisions of the National Standard for Food Safety Strain Preparations for Food Processing (GB 31639-2023).
