With the development of science and technology and changes in market demand, the development and application of new food ingredients are becoming more and more frequent. China’s “Administrative Measures for the Review of the Safety of New Food Ingredients” makes it clear that new food ingredients refer to the following items that have no traditional consumption habits in China:
(i) Animals, plants and microorganisms;
(ii) Components isolated from animals, plants and microorganisms;
(iii) Food ingredients whose original structure has been changed;
(iv) Other newly developed food ingredients, the safety assessment and approval procedures of which shall strictly follow the provisions of the Administrative Measures for the Review of the Safety of New Food Ingredients. Among them, “termination of review” as one of the conclusions of the review of new food ingredients, involving a variety of specific circumstances, food partner network according to the National Health Commission issued the termination of the review of new food ingredients summarizes the “termination of the review” of the different circumstances that may be involved, for business For enterprises’ reference.
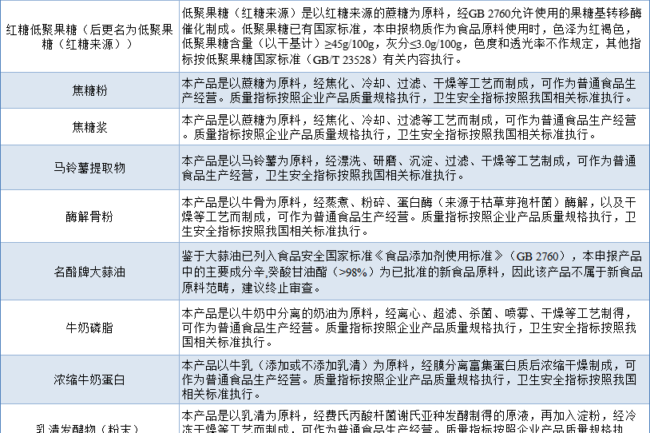
Circumstances categorized as common food management (33 kinds)
One of the cases of termination of review for substances to be declared as new food ingredients is categorized as common food management, which mainly includes that the raw materials can be verified as common food, or have substantial equivalence with existing common food, and may already have relevant national standards, or products with a long history of consumption. These ingredients can be legally produced and sold as common foods, or they can be used as food ingredients in a wide range of food products.
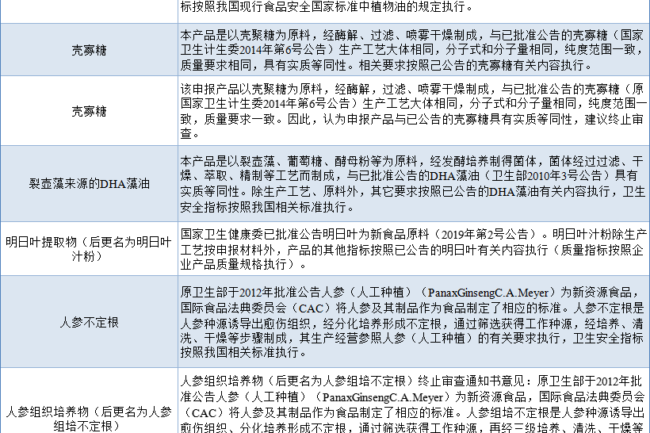
Substantial equivalence to approved new food ingredients (24 cases)
The substance to be declared as a new food ingredient is consistent with the announced new food ingredient in terms of species origin, biological properties, main components, edible parts, usage amount, scope of application and application population, etc. The process and quality requirements adopted are basically the same, indicating that the two are substantially equivalent in terms of safety. In this case, the raw material will be implemented in accordance with the management regulations of the approved new food ingredients, and its use must be in strict accordance with the content of the announcement and the relevant standards.
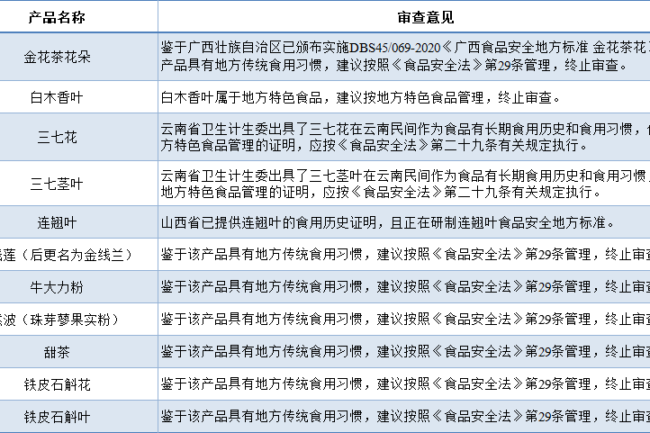
Circumstances to be managed as local specialty food (11 kinds)
For some food ingredients with distinctive traditional consumption habits within specific regions and recognized by local food safety regulatory authorities, even if they are not commonly consumed nationwide, they may be included in the termination of the review catalog due to their unique local characteristics and transferred to the special management of foods with local characteristics. This means that these ingredients may be used directly as legitimate food ingredients within the province, and provincial food manufacturers must manufacture their products in strict compliance with provincial food safety local standards. If enterprises in other provinces plan to use such local characteristics of raw materials, there may be regulatory adaptability and safety risks, it is recommended to actively communicate with the local food safety regulatory authorities to obtain professional guidance and approval, to ensure that the process of cross-regional application of national laws and regulations and food safety standards, to protect the rights and interests of consumers and the standardized development of the market.
Management as medicinal and food homologous substances (4 kinds)
In China, medicines and foods with the same origin refer to those traditional substances that are both Chinese herbs and foods. If the substance to be declared as a new food ingredient possesses the attribute of being homologous to medicine and food, or is substantially equivalent to the substance in the published list of homologous substances, the reviewing department may terminate the review of the new food ingredient in accordance with the relevant regulations and manage it directly as a substance of homologous origin to medicine and food.

Other cases of termination of review (1 type)
In addition, there are some other special circumstances for the termination of review of substances to be declared as new food ingredients, such as having been included in the Pharmacopoeia with clear pharmacological activity, which may make it clear that the substance is not a food ingredient.

In the approval process of new food ingredients, “termination of review” does not mean the end, but is an indirect way for enterprises to obtain approval. Even if it is not “recommended for approval”, if the declared raw material is substantially equivalent to existing food ingredients, or has a long history of consumption and is suitable to be included in the management of general food or local specialty food, etc., it can pass the “termination of review” procedure and be categorized into the corresponding categories, such as substantially equivalent to existing new food ingredients, or with long history of consumption and suitable to be included in the management of general food or local specialty food. All of them can pass the procedure of “termination of examination” and be classified into the corresponding categories such as substantially equivalent to existing new food materials, managed as general food, managed as local specialty food or food with medicinal origin, etc., and legally enter the market circulation.
Therefore, no matter through the regular “recommendation for approval” or through the “termination of review” mechanism, enterprises can obtain the market access qualification of new food ingredients, which provides enterprises with more choices and flexibility in product development and market expansion.
