What is the difference between alpha-amylase, beta-amylase, and glycosylated amylase?
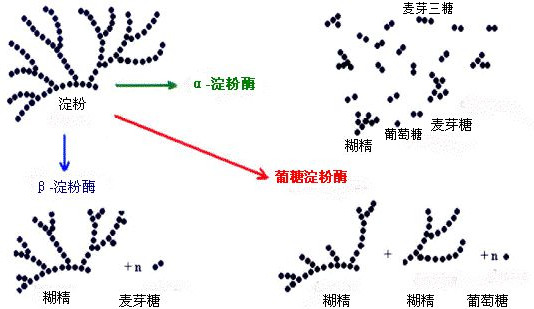
According to the role of α-amylase, β-amylase and saccharification amylase diagram is easy to let a person mistakenly think that the role of the three substrates must be starch, dextrin and maltose, respectively, and there are children’s shoes once asked me saccharification amylase is not maltose enzyme. Therefore, I feel the need to do a little analysis of the role of the three.
In order to understand the role of several types of amylases, first need to identify two groups of concepts: straight chain starch and branched chain starch, α-1,4-glycosidic bond and α-1,6-glycosidic bond.
There are two types of starch: straight chain starch and branched chain starch. Straight-chain amylopectin is an unbranched helical structure, consisting of glucose residues linked only by α-1,4-glycosidic bonds; branched-chain amylopectin is formed by glucose residues linked by α-1,4-glycosidic bonds in each chain, but α-1,6-glycosidic bonds are present at the branching points. α-Amylase and its action
α- Amylase, also known as endo amylase, is a metallohydrolase that requires calcium ions, which bind to the enzyme protein to exhibit activity, which can be lost by treatment with the chelating agent EDTA.α- Amylase is a stoichiometric amylase that is capable of randomizing its activity.
α- Amylase can randomly hydrolyze the α-1,4-glycosidic bond within starch, but cannot hydrolyze the α-1,6-glycosidic bond.
If the substrate is straight-chain starch, the hydrolysis produces glucose, maltose, and maltotriose; if the acting substrate is branched-chain starch, the hydrolysis products are glucose, maltose, maltotriose, and α-dextrin containing α-1,6-glycosidic bonds containing more than three glucose residues. It should also be noted that the α and β in α-amylase and β amylase do not indicate any conformational relationship, but are simply numbered.
There are many ways to produce α-amylase in nature, for example, microbial fermentation is one of the most common ways. In addition, this enzyme can also be extracted from plants or animals, and the properties of amylase obtained in different ways are different. In the industrial production process, due to the large demand for α-amylase, it is usually made by the fermentation of fungi and bacteria. Bacillus subtilis, Bacillus sphaericus, Streptomyces suis, etc. can produce α-amylase. β-Amylase and its role
β-amylase, also known as exo amylase, is a hydrolase containing sulfhydryl groups. The enzyme starts from the non-reducing end of starch and hydrolyzes the α-1,4-glycosidic bond sequentially with two glucose residues to produce maltose. It cannot hydrolyze the α-1,6-glycosidic bond, and it cannot cross the branching point and be left with a very long dextrin, i.e. β-dextrin.
Therefore, when β-amylase acts on straight-chain starches, it produces almost exclusively maltose; when it is used on branched-chain starches, the products are maltose and β-dextrin.
R-enzyme and its action
R-enzymes, also called debranching enzymes, act on the α-1,6-glycosidic bond. In the presence of debranching enzymes, the α-1,6-glycosidic bond of the α-dextrin and β-dextrin species is hydrolyzed, and the branching branched chains are removed.
The remaining straight chains are then hydrolyzed by α-amylase and β-amylase to produce maltose and glucose. However, R-amylases cannot directly hydrolyze the α-1,6-glycosidic bonds within branched starches.
Glycosylating amylase and its action
Glycosylating amylase, or glucoamylase, is an enzyme that hydrolyzes starch to glucose, where the percentage of hydrolysis can reach 100%, and is often used as a saccharifying agent for starch.
Glucoamylase is not very specific and is applicable to many substances, it can not only cut the α-1,4- glycosidic bond from the non-reducing end of the starch molecule, but also cut the α-1,6-glycosidic bond, just that the hydrolysis of the α-1,4-glycosidic bond is a little faster. It can also hydrolyze dextrin, maltose and glycogen. Hydrolysis is initiated from the end of the substrate molecule and is an exonuclease.
The results of the action of α-amylase, β-amylase, and glycolytic amylase on branched-chain starch can be represented in the following diagram: