What are new food ingredients? And what is the difference between new food ingredients and other foods?
In recent years, with the continuous improvement of the quality of life of the nation, people pay more and more attention to the health of individuals and their families, and at this time the concept of “new food ingredients” gradually into the public’s eyes, that in the end, what is the new food ingredients? New food ingredients and other food and what is the difference?
What is new food ingredients
With the change of China’s new food ingredients management system, the name of the new food ingredients from the “new resource foods” to “new food ingredients” change process, the connotation of the concept of new food ingredients has also changed.
On October 1, 2013, the Administrative Measures for the Review of the Safety of New Food Ingredients (formulated by the National Health and Family Planning Commission) came into force, and the Administrative Measures for New Resource Foods was repealed on the same day. Since then, the term new food raw materials has replaced the term new resource foods, and new food raw materials refer to the following items that are not traditionally consumed in China:
(a) animals, plants and microorganisms; (b) components separated from animals, plants and microorganisms; (c) food components whose original structure has been changed; (d) other newly developed food ingredients.
The “traditional eating habits” refers to a food in the provincial jurisdiction has more than 30 years as a stereotypes or non-stereotypes of food production and management of the history of packaging, and is not included in the “Pharmacopoeia of the People’s Republic of China”.
At the same time, this approach provides for new food ingredients should be consistent with the “Food Safety Law” and relevant laws, regulations, standards, shall not produce any acute, subacute, chronic or other potential health hazards to the human body.
The difference between new food ingredients and traditional food
First: new food ingredients are not habitually eaten, basically no one has eaten before, or did not take it as food;
Second: new food ingredients are listed for sale, you must apply to the health department for the record, the audit is approved before listing;
Third: most of the new food ingredients have specified consumption limits and consumption of people. The Administrative Measures for the Review of the Safety of New Food Ingredients stipulates that if a food product contains new food ingredients, its product labeling and identification should be in accordance with national laws, regulations, food safety standards and the requirements of the announcement of the National Health and Family Planning Commission. The use of new food ingredients must be used in accordance with the provisions of the announcement, and if the announcement has the requirement, it should be labeled in accordance with the requirements of the announcement of the unsuitable people and the consumption limit.
China’s first batch of new food ingredients (i.e., the original new resource food) recognized as early as 2004 began, one after another to now have more than 200 kinds of, are after a complex audit process, safety certification. Difference with health food
I. Health food refers to food with specific health functions, and application for approval must be clearly indicated with which health function, and the need for health function labeling and qualification on the product packaging (the so-called blue hat), while the new food ingredients with one or more functions are not in the product description of the detailed labeling.
II. New food ingredients and health food for different groups, the former is suitable for any group of people, while the latter is suitable for consumption by a specific group of people.
What are the new food ingredients?
If you want to know whether an ingredient is an approved new food ingredient, or you want to check which new food ingredients have been approved, terminated for review or open for comments, you can check through the “Public Query System for Health Administrative License of the National Health Commission”.
In this query system, it is divided into new resource foods approved before 2007 and new food ingredients approved after 2007 announcement catalog, new food ingredients terminated review catalog. Inquiry link: https://slps.jdzx.net.cn/xwfb/gzcx/PassFileQuery.jsp
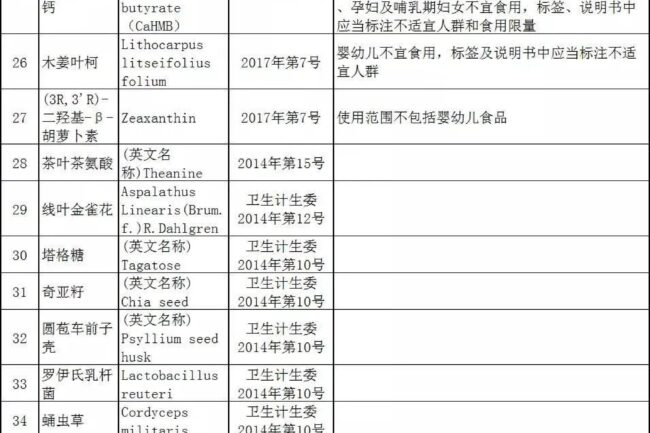
Catalog of New Food Ingredients (Partial)↓

Questions & Answers
Q1.What procedures should importers fulfill when importing food products for which there is no national standard for food safety, or when importing new varieties of food additives or new varieties of food-related products for the first time?
A: The import of food without national standards for food safety, should be consistent with the State Council administrative department of health announced the temporary application of the relevant standard requirements.
The use of new food raw materials for the production of food, should be in accordance with the provisions of Article 37 of the Food Safety Law, the State Council administrative department of health to obtain new food raw materials health administrative license.
According to the requirements of Article 37 and Article 93 of the Food Safety Law, the relevant application shall first be made to the health administrative department of the State Council.
Article 37 The use of new food raw materials for the production of food, or the production of new varieties of food additives, new varieties of food-related products, shall be submitted to the health administrative department under the State Council to assess the safety of the relevant products. Health administrative department under the State Council shall receive the application within sixty days from the date of review of the organization; to meet the food safety requirements, permission is granted and announced; does not meet the food safety requirements, not permitted and a written statement of reasons.
Article 93 The import of food safety standards are not yet national standards, by overseas exporters, overseas manufacturers or their commissioned importers to the State Council administrative department of health to submit the implementation of the relevant national (regional) standards or international standards. Health administrative department of the State Council to review the relevant standards, that meet the food safety requirements, the decision to apply temporarily, and the timely development of the corresponding national food safety standards. The import of food produced using new food raw materials or import new varieties of food additives, new varieties of food-related products, in accordance with the provisions of Article 37 of this Law.
Q2. imported new food ingredients must be in the country of origin to allow the production or sale?
A: Yes, according to the “Declaration and Acceptance of New Food Ingredients Regulations” Article VIII: Application for the import of new food ingredients, in addition to the submission of the materials specified in Article VII, the following materials should also be submitted: (a) the import of new food ingredients exporting country (region) of the relevant departments or agencies to allow the production of the product in their own country (region) production or sale of certificates; (b) the import of new food ingredients production enterprises Where the country (region) relevant institutions or organizations issued by the producer of the review or certification materials. This provision only regulates the import of raw materials, for the use of the product is not clear.
Q3. Is it necessary to fill in the registration number of overseas manufacturers in China for the declaration of imported new food ingredients or food containing new food ingredients?
A: Yes, for food products shipped to China since January 1, 2022, the registration number of the enterprise in China should be filled in the certificate column of “Registration of Overseas Manufacturing Enterprises of Imported Food Products” under “Product Qualification” in the customs declaration form (License Category Code 519). No.
For imported food products declared at the Customs of the implementation of the 2020 version of the declaration program, “overseas production enterprises of imported food products” should be selected in the column of “Other Enterprises” under the item of “Other Enterprises”. “No. or enterprise name” column fill in the enterprise registration number in China.
Customs will not accept the declaration if it is not filled out in accordance with the requirements.
Q4. Is it necessary to fill in the “new food ingredients license” and other regulatory documents, such as the name of the license, number and other relevant information?
A: No. According to Announcement No. 152 of 2019 jointly issued by the General Administration of Customs and the National Health Commission, since September 27, 2019, two types of regulatory documents (hereinafter referred to as documents), such as the “Permit Certificate for New Food Ingredients” and the “Temporarily Applicable Criteria for Importing Foods for Which National Standards for Food Safety Do Not Yet Exist”, have been withdrawn from the port inspection and verification.
