What are safe and effective natural antioxidants?
The deterioration of food, in addition to the role of microorganisms and the occurrence of corruption and deterioration, but also and the oxygen in the air oxidation reaction occurs, resulting in food fat rancidity, discoloration, browning, flavor deterioration and vitamin destruction, and even produce harmful substances, thereby reducing the quality of food and nutritional value. Accidental consumption of such foods may even cause food poisoning and jeopardize human health. Adding antioxidants to food can prevent food from oxidizing and deteriorating.
Classification of Food Antioxidants
At present, there is no uniform standard for the classification of food antioxidants. As the basis of classification is different, it will produce different classification results. According to the source, they can be divided into synthetic antioxidants (e.g. BHA, BHT, PC, etc.) and natural antioxidants (e.g. tea polyphenols, phytic acid, etc.).
According to solubility, they can be divided into three categories: oil-soluble, water-soluble and partially soluble. Oil-soluble antioxidants include BHA, BHT, etc. Water-soluble antioxidants include vitamin C, tea polyphenols, etc. Partially-soluble antioxidants include ascorbyl palmitate.
According to the mode of action can be divided into free radical absorbers, metal ion chelators, oxygen scavengers, peroxide decomposers, enzyme antioxidants, ultraviolet absorbers or single-linear oxygen quencher.
Mechanism of action of food antioxidants
Due to the more types of antioxidants, antioxidant mechanism is not the same, summarized, there are mainly the following: 1, through the reducing effect of antioxidants, reduce the oxygen content of the food system; 2, interrupt the oxidation process of the chain reaction, preventing the oxidation process further; 3, destruction, weakening of oxidative enzymes, so that it can not catalyze oxidation reaction; 4, will catalyze and cause oxidation reaction of the substances closed, such as complexes, and the oxidation reaction is caused by the oxidant. 4, will be able to catalyze and cause the oxidation reaction of the material closed, such as complexation of metal ions that can catalyze the oxidation reaction. The following automatic oxidative rancidity of fats and oils and food enzymatically oxidized browning as an example of the role of antioxidants to be briefly introduced to the mechanism.
2.1 Antioxidants inhibit the oxidation of fats and oils, natural fats and oils exposed to the air will spontaneously undergo oxidation reactions, generating low-grade fatty acids, aldehydes, ketones, etc., resulting in a bad sour smell and taste deterioration, etc., which is the main reason for the deterioration of fats and oil-containing foods. The automatic oxidation of fats and oils follows the free radical (also called free radical) reaction mechanism, first of all, the fat molecule (expressed as RH) is activated by heat, light, or metal ions and other radical initiators, and then decomposed into unstable radicals R- and H-.
When molecular oxygen is present, the free radical reacts with oxygen to form a peroxide radical, and this peroxide radical reacts with the fat molecule to form hydroperoxide and radical R-, which is then passed on through the chain reaction of radical R- until the combination of free radicals and free radicals or free radicals and free radical inactivators (denoted by x) produces a stabilized compound, at which point the reaction is terminated.
This process produces many short-chain carbonyl compounds such as aldehydes, ketones, and carboxylic acids, which are the main substances that produce rancidity and poor taste, and the presence of large quantities of peroxides, which can also have undesirable results on the human body. The mechanism of action of antioxidants is most notably to terminate the transmission of the chain reaction in the following pattern (with AH denoting antioxidants):
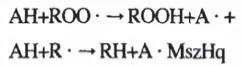
The free radical A- of antioxidants is inactive, it cannot cause chain reaction but can participate in some termination reactions. For example: A- A-→AA A- ROO-→ROOA_Fats and oils antioxidants are mainly butylhydroxyanisole (BHA), dibutylhydroxytoluene (BHT), propyl gallate (PG), tert-butylhydroquinone (TBHQ), tocopherol (Vitamin E) and so on, which all belong to the phenolic antioxidants, and they are more stable after formation of the free radicals, and the reason for this can be explained as follows: the oxygen atom on the The reason for this can be explained by the fact that the unpaired electrons on the oxygen atom can interact with the π-electron cloud on the benzene ring, and a conjugation effect occurs. The result of this conjugation is that the paired electrons are not fixed on the oxygen atom, but are partially distributed to the benzene ring. In this way, the energy of free radicals is reduced, no longer triggering chain reactions, and playing an antioxidant role.
2.2 Inhibition of Enzymatic Oxidative Browning of Foods Enzymatic oxidative browning is a class of reactions in which phenol oxidase catalyzes the oxidation of phenolic substances in food to form quinones and their polymers. As the reaction generates melanin-like substances, the color of the food is deepened, thus affecting the appearance quality of the food.
Enzymatic oxidative browning requires three conditions: phenol oxidase, oxygen, and appropriate phenolic substances, all of which are indispensable. Therefore, the inhibition of enzymatic oxidative browning of food can be considered from these three conditions. Since the possibility of removing phenolic substances from food is small, the main measures that can be used are to destroy and inhibit the activity of phenol oxidase and to eliminate oxygen. Adding the appropriate amount of antioxidants to food can prevent enzymatic oxidative browning of food by consuming the oxygen in the food system through reduction. Research on natural antioxidants
As consumers’ requirements for food safety improve and doubts about the safety incidents of chemical synthetics increase, people are looking forward to the development of natural antioxidants that are safe and highly effective. The varieties of natural antioxidants permitted for use in China are increasing. In addition to vitamin E (tocopherol), which is included in food fortification, there are currently ascorbic acid series, tea polyphenols, phytic acid, licorice antioxidants, ceruloplasma, rosemary extracts, etc., and in particular, the amount of sodium isoascorbate is rapidly increasing year by year.
3.1 Vitamin C, also known as L-ascorbic acid, referred to as VC, is white or slightly yellowish crystals or powder, odorless, acidic taste, melting point of 190 ~ 192 ℃ (decomposition). Easily soluble in water, dry state is more stable, but the aqueous solution is easily oxidized and decomposed, especially in neutral or alkaline solution; heavy metal ions can promote its oxidation and decomposition, the color gradually becomes darker when exposed to light, so it should be protected from light and kept airtight.
This product can combine with oxygen to become a deoxidizer and inhibit the oxidation of oxygen-sensitive food ingredients; it can reduce high-valent metal ions and play a synergistic role in chelating agent; it has the effect of treating scurvy, detoxification and maintaining capillary permeability. At present, the main production processes are natural substance extraction method, Lay’s method and twice fermentation method.
In practical use, this product can be applied to many food products, including fruits, vegetables, meat, fish, beverages and fruit juices. Applied to cured meat products, ascorbic acid as a coloring additive, 0.02% to 0.05% of the amount added, can effectively promote the production of meat red nitrosomyoglobin, to prevent the discoloration of meat products, and at the same time, inhibit the generation of carcinogenic nitrosamines. Applied to fruit juice and carbonated beverage, the addition amount of 0.005%~0.002% can effectively prevent the beverage from discoloration and flavor change. Applied to fruit and vegetable processing, mainly used to inhibit browning, maintain flavor and color.
3.2 Tea polyphenols tea polyphenols are a class of polyhydroxyphenolic compounds contained in tea, referred to as TP, the main chemical composition for the catechins (flavanols), flavonoids and flavonols, anthocyanins, phenolic acids and phenolic acids, polymerization of phenols and other compounds of the complex. Among them, catechin compounds are the main components of tea polyphenols, accounting for about 65% to 80% of the total amount of tea polyphenols. Catechin compounds mainly include catechin (EC), gallocatechin (EGC), catechin gallate (ECG) and gallocatechin gallate (EGCG) 4 kinds of substances.
Tea polyphenols have strong antioxidant effects, especially ester-type catechin EGCG, whose reducibility can even be up to 100 times that of VC. 4 main catechin compounds, the antioxidant capacity of EGCG>EGC>ECG>EC>BHA, and antioxidant performance with the increase in temperature and enhancement of antioxidant performance, the antioxidant effect of the animal oils and fats is better than that of the vegetable fats and fats, and with VE, VC, lecithin, It can be used together with VE, VC, lecithin, citric acid, etc. It has obvious synergistic effect and can also be used jointly with other antioxidants.
3.3 Phytic acid, also known as cyclohexanol hexakisphosphate, inositol hexakisphosphate, referred to as PA, yellow to yellow-brown viscous towel-like liquid, aqueous solution of strong acidity, easy to decompose at high temperatures. Phytic acid has a strong antioxidant capacity, mixed with VE, with multiplying antioxidant effect. Phytic acid molecule in the 12 acid hydroxyl can be chelating metal ions, in the low pH can be quantitative precipitation of iron ions, medium pH or high pH can be with all the other polyvalent metal ions to form insoluble chelates.
China’s “Hygienic Standard for the Use of Food Additives” (GB2760-2014) stipulates that: for shrimp preservation, it can be used in moderation according to production needs, and the allowable residue is 20mg/kg; for edible fats and oils, fruit and vegetable products, beverages and meat products, the maximum use of 0.2g/kg.In practice, phytic acid is often used as an antioxidant and a chelating agent of metal ions, which is used for the prevention of oxidation of food, browning or discoloration of food.
Add 0.01% in vegetable oil can obviously prevent the rancidity of vegetable oil; for canned aquatic products, phytic acid can prevent the formation of guano crystals and discoloration, add 0.1% to 5% of phytic acid can prevent canned shellfish black; add 0.1% of phytic acid and 1% of sodium citrate can prevent the canned crab blue spots; add 0.01% to 0.05% of phytic acid and 0.3% of sodium sulfite to prevent fresh shrimp from turning black; add 0.01% to 0.05% of phytic acid and 0.3% of sodium sulfite to prevent fresh shrimp from turning black. Sodium sulfite, to prevent fresh shrimp blackening effect is very good, and can avoid excessive sulfur dioxide residue, phytic acid can also be used for fruits and vegetables preservation, by the cucumber, tomatoes, bananas and other experiments have obvious effect; for meat products, phytic acid can chelate its myoglobin in the iron, to prevent fat oxidation caused by iron catalyst; phytic acid can be used in the wine industry to remove the metal agent, water softeners and health care beverages, and the rapid thirst quencher. Quick thirst-quenching agent.
3.4 Rosemary rosemary, (Latin name: Rosmarinus officinalis) the labiatae family shrub. Sex like warm climate, native to the European region and the Mediterranean coast of northern Africa. As far away as in the Cao Wei period had been introduced to China. From the flowers and leaves of rosemary can be extracted antioxidants with excellent antioxidant properties and rosemary essential oil.
Rosemary antioxidant is widely used in medicine, fried food, oil-rich food and all kinds of fats and oils to preserve freshness and quality; and rosemary essential oil is used in spices, air fresheners, ant repellents, as well as bactericidal, insecticidal and other daily-use chemical industry.
Rosemary extracts contain a variety of active ingredients such as sage acid, rosemary acid, sage phenol, etc., which not only has the antioxidant effect that traditional plant extracts have, but also has more antiseptic and antibacterial activities. In addition, rosemary extract also has the property of high temperature resistance: at 240℃, it is still very stable, while the stability of general plant extracts is easily affected by temperature.
Rosemary is a natural antioxidant herb. In a recent study, Dr. Stuart A. Lipton of the Sanford-Burnham Medical Research Institute in the United States and his colleagues reported that carnosic acid, a component of the herb rosemary, can promote eye health. Their findings suggest that carnosic acid may have clinical applications for diseases affecting the outer retina, including age-related macular degeneration.
Rosmarinic acid (RosA) is a water-soluble natural phenolic acid compound isolated from rosemary, which is more widely distributed, mainly found in a variety of plants in Labiatae, Comfrey, Cucurbitaceae, Tiliaceae, and Umbelliferae, with the highest content in Labiatae and Comfrey, in particular.
Rosemarinic acid is a natural antioxidant with stronger antioxidant activity than vitamin E, caffeic acid, chlorogenic acid, folic acid, etc. It helps prevent cell damage caused by free radicals. Since rosemary extract is insensitive to light and heat as well as acids, it is recognized as a natural antioxidant by the industry because of its antioxidant effects in food supplements, food and beverages, fresh meat, and even seasonings. Problems and Prospects in the Industrialization of Natural Antioxidants
4.1 Sources of natural antioxidants Discover new sources of raw materials in some medicinal and food plants. It is of great significance for the further industrialization of natural antioxidants to obtain materials with higher antioxidant active substances through cultivation, introduction and screening.
4.2 Extraction and purification of natural antioxidants In the process of extracting and separating antioxidants from biological materials, the complexity of biological materials will interfere with the extraction effect, so they are often extracted by using a large number of organic solvents or strong acid and strong alkali solutions, but the antioxidant components of the extracts have a low purity, which not only cause environmental pollution but also increase the cost. It is not conducive to the industrial production of natural antioxidants. Therefore, it is necessary to study the new technology of extraction and purification of antioxidant functional components.
Through the application of physical field and enzyme-assisted extraction technology, supercritical extraction, membrane separation, large soliton resin adsorption, chromatographic separation and other technologies, it is necessary to establish a highly efficient extraction and purification method of antioxidants, improve the cleanliness of the production process, and promote the development of circular economy.
4.3 Antioxidant synergistic effect At present, natural antioxidants are mostly in the form of monomers in the market. Some studies have shown that the antioxidant activity of monomer is often not as high as the antioxidant activity of multi-component Yan. For example, Wang Shaomei et al. found that tea polyphenols and vitamin C have synergistic antioxidant effects in lard emulsification system. Natural antioxidants generally have other physiological functions in addition to the efficient antioxidant function. Composite natural antioxidants can give full play to a variety of physiological functions, and if they can be produced industrially, they will be of great significance to human health.
