What is the protective effect of erythritol on vitamin C in lemonade drinks?
Vitamin C in fruit juice is one of the important nutrients needed by human body in daily life, but due to its instability, it is easy to receive the influence of storage environment, such as temperature, light, and the internal system of fruit juice PH and other conditions, which causes oxidation reaction and generates a large number of free radicals, thus resulting in the oxidative decomposition of vitamin C.
Erythritol, as a new type of natural sweetener promoted in recent years, is widely used in the beverage and confectionery industries due to its pure sweetness and no unpleasant bitter aftertaste, as well as its low calorie, high tolerance, and no blood glucose fluctuations caused by metabolism in the body and other biological characteristics.
At the same time, researchers have found that erythritol can also be used as an antioxidant, which can effectively remove free radicals and inhibit their generation. At present, domestic and foreign research on erythritol in beverages mainly focuses on its application as a new type of sweetener, this paper takes lemon juice as an example to explore the protective effect of erythritol on vitamin C in lemon juice in the morning.
Thermal degradation kinetics of vitamin C in lemon juice
Kinetic study of thermal degradation
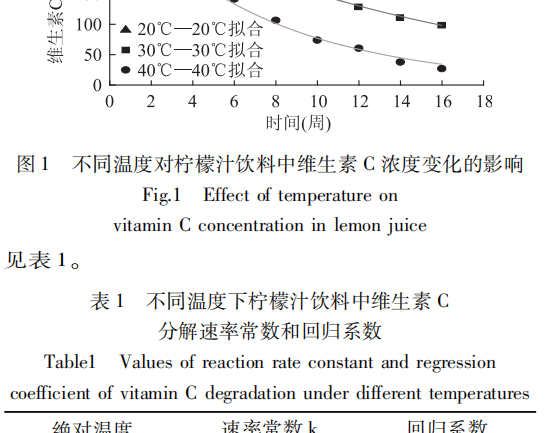
The trends of vitamin C content in lemon juice drinks with time at different temperatures were obtained by experimental determination and fitted, and the fitting results are shown in Fig. 1.
It can be seen from Fig. 1 that the content of vitamin C gradually decreased with the prolongation of the storage time of lemon juice beverage; and its decomposition rate was accelerated along with the increase of temperature. The preservation rate of vitamin C was 60.82%, 31.98, and 8.48% for 16 weeks of storage under the environmental conditions of 20, 30, and 40°C, respectively.
The fitting results showed that the decomposition of vitamin C in lemon juice drinks at different temperatures was modeled by a compound first-order reaction kinetic model, i.e:
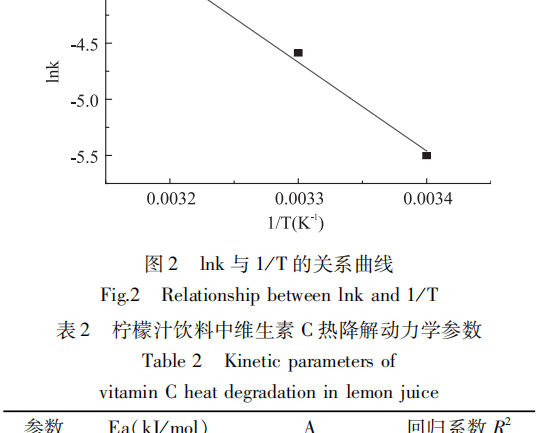
The decomposition rate constant k of vitamin C at different temperatures was calculated from Eq. (1) from the experimental data and analyzed by linear regression, which was obtained by taking the logarithm of the left and right sides of the Arrhenius equation at the same time:
According to the above equation (2), the logarithmic Ink of the rate constant of vitamin C primary reaction was plotted against the inverse 1/T left of the storage temperature, as shown in Fig. 2, and the activation energy Ea and the finger forward factor A were obtained from the slope and intercept of the straight line, respectively, and the results are shown in Table 2.
It is usually considered that the activation energy Ea of a chemical reaction is 40-400kJ/mol, and the smaller the activation energy Ea is, the easier the reaction is to proceed. When Ea < 42kJ/mol, the reaction rate is very large; when Ea > 400kJ/mol, the reaction rate is very small. From Table 2, it can be seen that the vitamin C of lemon juice beverage is easy to be degraded during the storage process.
The effect of erythritol on vitamin C
Effect of erythritol on the degradation rate of vitamin C
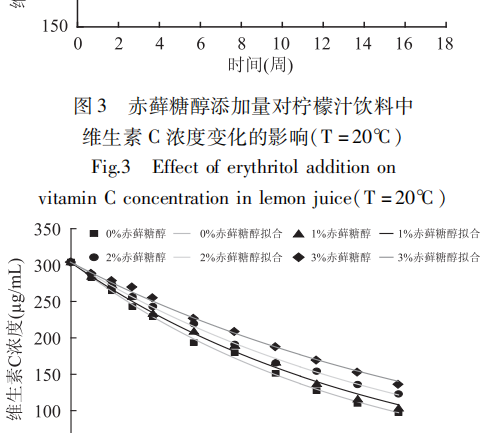
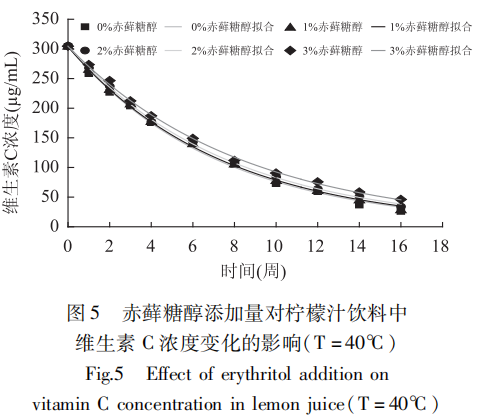
The effects of different erythritol additions on the vitamin C content of lemon juice beverages over time were determined experimentally at constant temperature and fitted to the trend, and the results were shown in Figures 3-5.
The fitting results showed that the degradation rate of vitamin C in lemon juice beverage with time under the above conditions was in accordance with the first-order reaction kinetic model. It can be seen from Figure 3-Figure 5 that the decomposition rate of vitamin C slowed down with the increase in the addition of erythritol under constant temperature, and the retention rates of vitamin C after 16 weeks of storage were all increased to different degrees relative to the control (no addition).
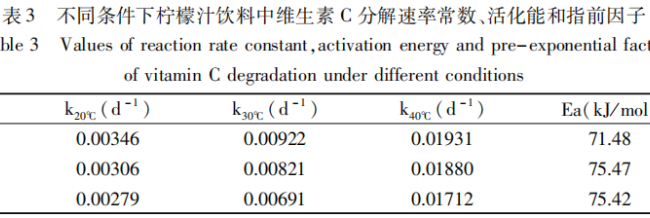
According to the above experimental data, the decomposition rate constants k and the corresponding activation energy Ea and finger forward factor A of vitamin C under different conditions were calculated by Eq. (1) and Eq. (2), and the results are shown in Table 2.
As shown in Table 2, the activation energies of vitamin C degradation reaction in lemon juice beverage after the addition of erythritol were higher than those of the samples without the addition of erythritol (65.68% kJ/mol), so the degradation reaction of vitamin C was difficult to be carried out in comparison with that of the control group and the activation energies basically showed an increasing trend with the increase of the concentration of erythritol added.
Among them, when the concentration of erythritol was 2%, the activation energy of vitamin C degradation reached a maximum of 75.47 kJ/mol, which was 9.79 kJ/mol higher than that of the control; however, with the increase of the concentration of erythritol, the activation energy of the reaction was basically unchanged.
In conclusion, the addition of erythritol to lemon juice beverage can indeed play a role in protecting vitamin C to a certain extent.