The mechanism of kaempferol inducing apoptosis of triple negative breast cancer cells through mitochondrial apoptosis pathway
Triple negative breast cancer (TNBC), as a special subtype of breast cancer, accounts for 15%~20% of the total number of breast cancer. It has the clinical characteristics of short overall survival period, high malignancy, strong invasion, high early recurrence rate, etc. Once metastasis occurs, the median survival period of patients is only 10~13 months. Due to the insensitivity of TNBC to endocrine therapy and targeted therapy, chemotherapy is the main treatment method. However, drug resistance and treatment-related side effects remain issues of concern. In recent years, the role of traditional Chinese medicine in the prevention and treatment of TNBC has been increasingly valued due to its unique theoretical system and expertise in individualized treatment plans. Our research group found that the addition and reduction of Chaihu Guizhi Decoction combined with capecitabine can inhibit the growth of subcutaneous transplanted tumor of triple negative breast cancer in nude mice through previous research. Further analysis using network pharmacology method showed that kaempferol (KA) is an important effective component in the addition and reduction of Chaihu Guizhi Decoction, which provides an experimental basis for us to use kaempferol to treat triple negative breast cancer.
Kaempferol is a flavonoid compound widely found in vegetables, fruits, and traditional Chinese medicine. It has various effects such as anti-cancer, anti-inflammatory, antioxidant, and antiviral. It can inhibit the proliferation and invasion of tumor cells by inducing cell apoptosis, regulating the cell cycle, inhibiting angiogenesis, and tumor metastasis. Research has found that KA induces growth inhibition and apoptosis in lung cancer cells by activating MEK-MAPK. In vitro and in vivo, KA inhibits the AKT/PI3K and ERK pathways, activates the mitochondrial apoptosis pathway, and increases radiation killing of lung cancer cells. In colon cancer, KA reconstructs intercellular communication of gap junctions by enhancing the expression and phosphorylation of junction protein 43 in colon cancer cells, thereby inducing cell apoptosis. KA can also induce apoptosis and aging in human cervical cancer cells by downregulating the PI3K/AKT and hTERT pathways. In breast cancer, it has been reported that KA plays an anti-tumor role through different mechanisms. KA can inhibit the invasion of breast cancer cells by blocking the expression and activity of PKC δ/MAPK/AP-1 cascade and subsequently matrix metalloproteinase-9 (MMP-9). Li et al found that KA can inhibit the proliferation of breast cancer SK-BR-3 cells by regulating Notch1 protein. In addition, KA can also inhibit the epithelial mesenchymal transformation (EMT) and metastasis related behaviors of MCF-7 breast cancer cells induced by triclosan. Although the anticancer effect of KA in a variety of tumors has been confirmed, there is no report on whether KA can induce apoptosis of triple negative breast cancer cells and its molecular mechanism. This study will take human TNBC cell MDA-MB-231 as the research object to observe the effect of kaempferol on MDA-MB-231 cell apoptosis and whether the possible mechanism is related to the mitochondrial apoptosis pathway, so as to provide theoretical basis and experimental basis for clinical use of kaempferol in the treatment of triple negative breast cancer.
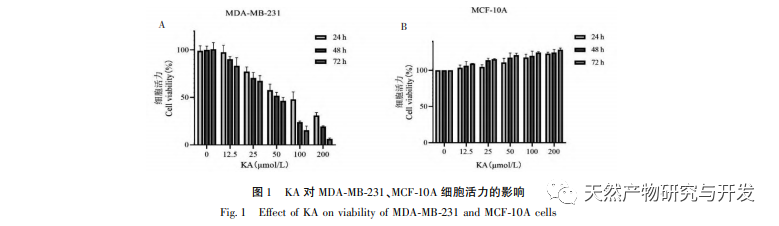
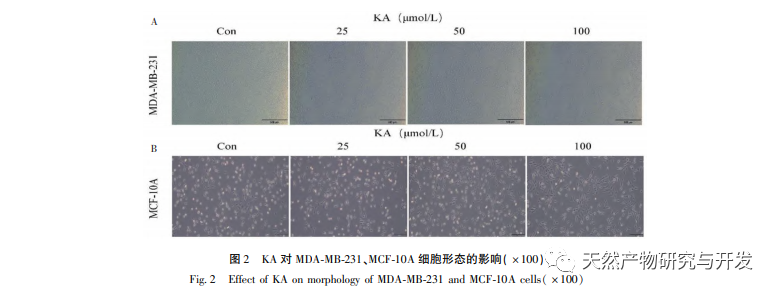
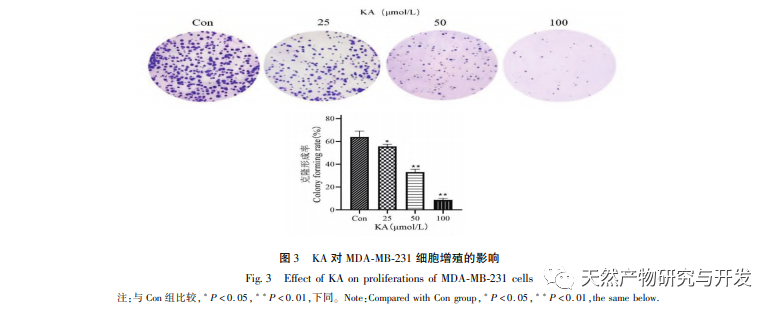
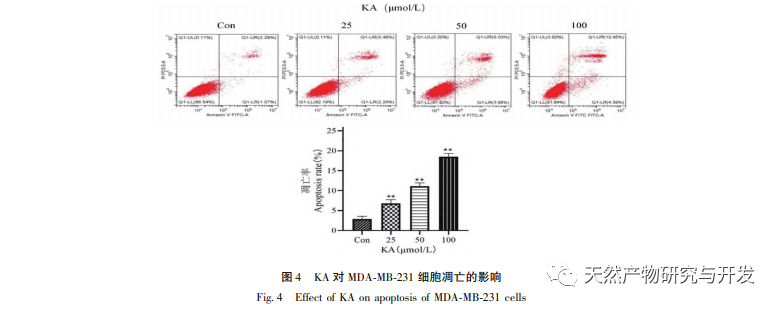
Kaempferol, as a widely present polyol hydroxy flavonoid compound in various plants, can not only effectively inhibit tumor cell growth and induce apoptosis, but also maintain the growth vitality of normal cells. Chen et al. found that KA can induce prostate cancer cell cycle arrest, inhibit cell proliferation, and have no effect on the viability of normal human foreskin fibroblasts (HFF), indicating that KA has good anti-tumor efficacy and low toxicity. Tu et al. found that KA can effectively inhibit the proliferation and metastasis of human cervical cancer cells, while its cytotoxic effect on carcinoembryonic kidney cells and normal liver cells is relatively small, indicating that KA has high cancer cell targeting ability. There are few studies on KA in triple negative breast cancer cells. Therefore, this study confirmed that KA can significantly inhibit the proliferation of TNBC cells and promote cell apoptosis through in vitro studies on MDA-MB-231 and MCF-10A cells, but has no effect on the proliferation of normal breast epithelial MCF-10A cells.
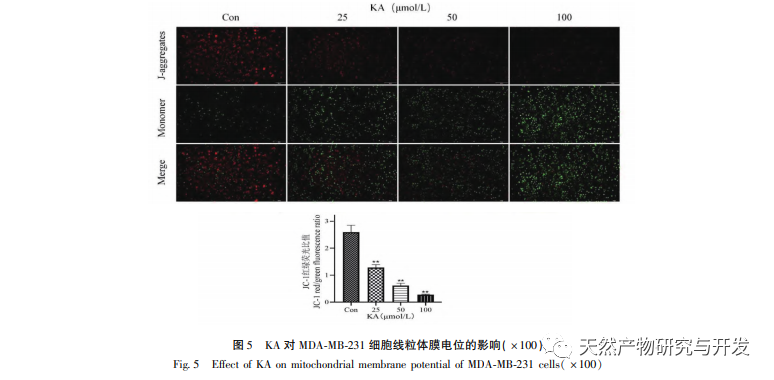
On this basis, this experiment further explores the apoptosis induction mechanism of KA on MDA-MB-231 cells. At present, research has found that cell apoptosis mainly involves three pathways based on the initiation process: mitochondrial pathway, death receptor pathway, and endoplasmic reticulum pathway, and the mitochondrial pathway plays an important role in mediating cell apoptosis. Mitochondrial membrane potential is one of the best indicators reflecting the permeability of the mitochondrial inner membrane. The decrease in mitochondrial membrane potential is considered to be the earliest event in the cascade reaction of cell apoptosis. Once the mitochondrial membrane potential collapses, cell apoptosis becomes irreversible. Feng et al. isolated an arabinogalactan from Artemisia scoparia, which can kill human nasopharyngeal carcinoma CNE-2 cells by causing mitochondrial membrane potential loss. This study found that KA has the effect of reducing mitochondrial membrane potential in MDA-MB-231 cells, suggesting that one of the mechanisms by which kaempferol inhibits cell proliferation and promotes apoptosis may be achieved through the mitochondrial mediated apoptotic pathway. In order to further investigate the relationship between KA induced apoptosis of MDA-MB-231 cells and mitochondrial apoptosis signaling pathway, this study detected the expression of Bcl-2, Bax, CytC, Cyclin D1, as well as the activity of Caspase-3 and Caspase-9.
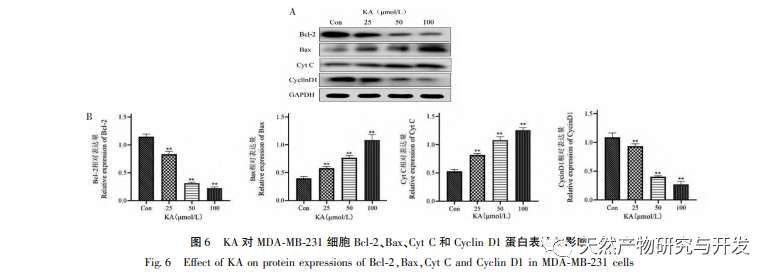
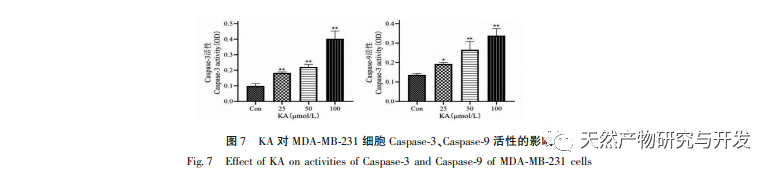
The Bcl-2 family of proteins includes the anti apoptotic protein Bcl-2 and the pro apoptotic protein Bax. As regulators of mitochondrial outer membrane integrity, they play an important role in regulating the mitochondrial apoptosis pathway. After receiving the apoptotic signal, Bax in the cytoplasm translocates to the mitochondria, and the anti apoptotic protein Bcl-2 guides the release of cytochrome C from the mitochondria, promoting the self activation of Caspase-9 precursor. Activated Caspase-9 activates Caspase-3, ultimately leading to cell apoptosis. Liu et al. found that KA inhibits the proliferation of human gallbladder cancer cells and induces their apoptosis through the mitochondrial apoptosis pathway. In this study, we observed that KA increased the expression of Bax and decreased the expression of Bcl-2 in MDA-MB-231 cells, promoting the release of CytC into the cytoplasm through the mitochondrial apoptosis pathway, resulting in increased expression. At the same time, mitochondrial membrane permeability increases, membrane potential decreases, pre apoptotic factors are released, triggering downstream activation of Caspase-3 and Caspase-9, ultimately leading to cell apoptosis. In addition, apoptosis is closely related to abnormal cell proliferation, and the main cause of cell proliferation is abnormal cell cycle. Cyclin D1, as an important regulatory protein of the cell cycle, can promote the transition of cells from G1 phase to S phase, accelerate the cell cycle process, and its upregulation can cause abnormal cell proliferation, induce changes in mitochondrial membrane potential, and lead to tumor development. The results of this experiment indicate that KA can downregulate the expression of apoptosis related proteins Cyclin D1 and Bcl-2 in MDA-MB-231 cells, reduce mitochondrial membrane potential, inhibit cell proliferation, and promote cell apoptosis.
To sum up, this study has clarified the anticancer effect of kaempferol on triple negative breast cancer cells in vitro, and has certain safety, and clarified the mechanism of KA inducing apoptosis of MDA-MB-231 cells through mitochondrial apoptosis signal pathway. Later, the inhibitory effect of KA on TNBC can be further studied through in vivo experiments, providing experimental basis for the development and clinical application of KA.
