Study on the therapeutic effect and mechanism of Cistanche deserticola phenylethanoid glycoside on chronic high altitude disease model rats
High altitude disease is a general term for various clinical manifestations caused by the body’s inability to adapt to low-pressure hypoxia at high altitudes, resulting in a series of pathological and physiological changes in the body. This disease is generally divided into two categories: acute and chronic. Chronic mountain sickness (CMS) refers to a condition where the original symptoms of acute mountain sickness persist or occur more than six months after arriving at high altitude. Researchers have found that the Egl9 homolog 1 (EGLN1) and peroxisome proliferator activated receptor alpha (PPAR – α) genes in the Tibetan population are different from those in low altitude areas, and can inhibit hemoglobin in the Tibetan blood, keeping it at a low concentration. This is also part of the reason why Tibetans can survive at high altitudes. This study suggests that the two genes mentioned above are candidate genes in the hypoxia response pathway, ultimately exerting their effects through the hypoxia inducible factor 1 alpha (HIF-1 alpha) pathway. EGLN1 is the upstream gene of HIF-1 α, while PPAR – α is the downstream target of HIF-1 α.
Low oxygen can block the hydroxylation and acetylation of HIF-1 α, increase its stability, and induce gene transcription information transmission by regulating the transcription and expression of downstream genes such as vascular endothelial growth factor (VEGF), endothelin-1 (ET-1), etc., thereby causing pulmonary vascular remodeling and further leading to pulmonary edema and high-altitude heart disease. Contrary to the action of ET-1, nitric oxide (NO) is a gas signaling molecule that catalyzes the production of L-arginine by nitric oxide synthase (NOS) and has a wide range of effects.
This study used a special environment artificial experimental cabin in the northwest to simulate the low oxygen environment of the plateau. Taking into account the main differences between the plateau and plain areas, a high-altitude disease animal model was established to investigate the role and intrinsic relationship of the HIF-1 α pathway in the pathogenesis of high-altitude disease. Based on this, the preventive and therapeutic effects of phenylethanoid glycosides from Cistanche (PhGCs) on high-altitude disease and their mechanisms were observed, providing a theoretical basis for studying the pathogenesis of high-altitude disease and developing new anti high-altitude disease drugs.
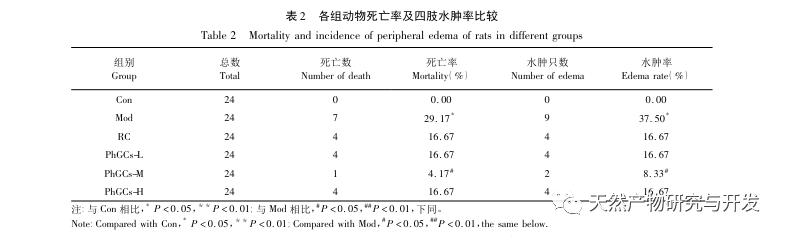
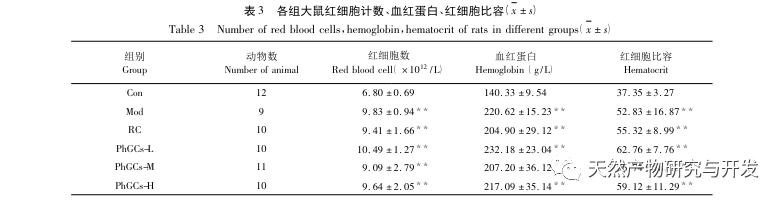

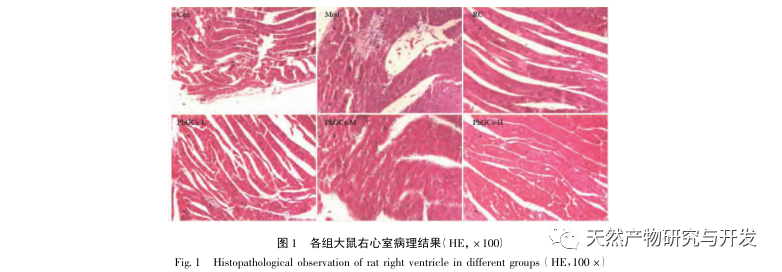
 PhGCs were extracted from Cistanche deserticola in southern Xinjiang, and various phenylethanolic glycosides were successfully isolated through related research, including echinacoside, verbascoside, isoverbascoside, 2 ‘- acetyl verbascoside, Cistanche deserticola glycoside A, and salidroside. For many years, in-depth research has been conducted on the pharmacological effects of PhGCs, such as antioxidant, free radical scavenging, and anti-aging. In this experiment, the PhGCs-M group was able to significantly reduce the right ventricular hypertrophy index in rats, indicating that Cistanche deserticola phenylethanolic acid can improve the hemodynamics and degree of right ventricular hypertrophy in CMS rats, and has a certain therapeutic effect on CMS. This study speculates from indicators related to blood oxygen saturation that PhGCs increase blood oxygen saturation, reduce the damage of hypoxia to the body, and increase the body’s tolerance to hypoxia damage under the same red blood cell count and hemoglobin content, thereby achieving the goal of treating altitude sickness. After treatment with PhGCs, the mortality rate and incidence of limb edema in CMS rats significantly decreased, further demonstrating its effectiveness in treating CMS.
PhGCs were extracted from Cistanche deserticola in southern Xinjiang, and various phenylethanolic glycosides were successfully isolated through related research, including echinacoside, verbascoside, isoverbascoside, 2 ‘- acetyl verbascoside, Cistanche deserticola glycoside A, and salidroside. For many years, in-depth research has been conducted on the pharmacological effects of PhGCs, such as antioxidant, free radical scavenging, and anti-aging. In this experiment, the PhGCs-M group was able to significantly reduce the right ventricular hypertrophy index in rats, indicating that Cistanche deserticola phenylethanolic acid can improve the hemodynamics and degree of right ventricular hypertrophy in CMS rats, and has a certain therapeutic effect on CMS. This study speculates from indicators related to blood oxygen saturation that PhGCs increase blood oxygen saturation, reduce the damage of hypoxia to the body, and increase the body’s tolerance to hypoxia damage under the same red blood cell count and hemoglobin content, thereby achieving the goal of treating altitude sickness. After treatment with PhGCs, the mortality rate and incidence of limb edema in CMS rats significantly decreased, further demonstrating its effectiveness in treating CMS.
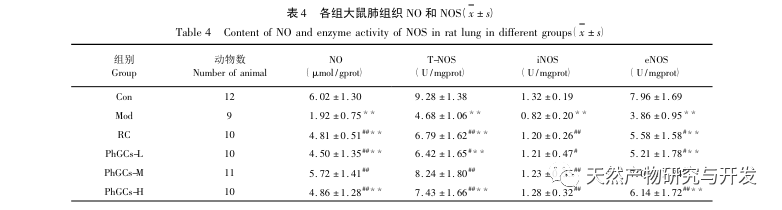
NO, as an endothelial dependent vasodilator, plays an important role in endothelial vasodilation because it directly relaxes pulmonary vascular smooth muscle, resulting in significant pulmonary vasodilation. It also has the ability to inhibit the proliferation and migration of pulmonary vascular smooth muscle cells, suppress platelet aggregation, and plays an important role in maintaining pulmonary circulation stability. This study found that the NO content in Mod lung tissue homogenate decreased, and the activities of T-NOS, iNOS, and eNOS decreased. PhGCs can increase the NO content in lung tissue by enhancing the activities of TNOS, iNOS, and eNOS, playing a role in combating the proliferation and myometrization of pulmonary artery smooth muscle and treating CMS.
ET-1 is the strongest vasoconstrictor discovered so far, and hypoxia can significantly increase the level of ET-1 in the blood. Rajput et al. compared the differences in ET-1 alleles between high-altitude and plain residents and found that certain alleles of ET-1 were overexpressed in high-altitude residents, and the expression of ET-l allele was significantly correlated with plasma ET-1 levels. In this article, the content of ET-1 in the lung tissue of Mod rats significantly increased, indicating that hypoxia induced increased secretion of ET-1 in lung tissue, consistent with other studies. Administration of PhGCs can decrease the content of ET-1 in the lung tissue of CMS rats, indicating that PhGCs can inhibit vascular constriction induced by ET-1, reduce pulmonary artery pressure, and exert therapeutic effects on CMS.
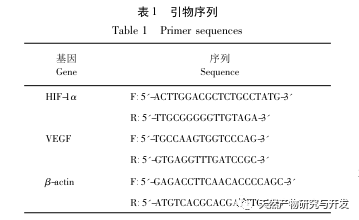
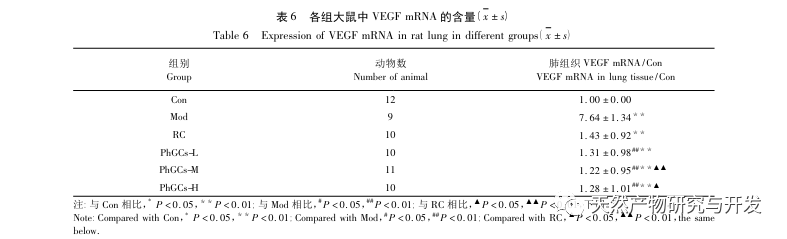
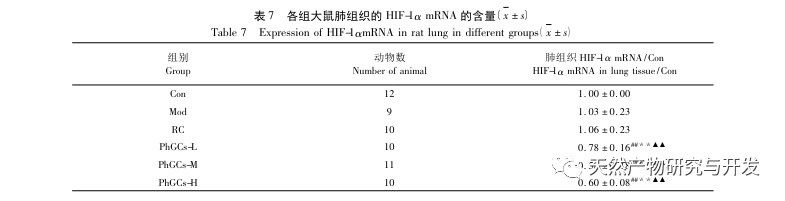
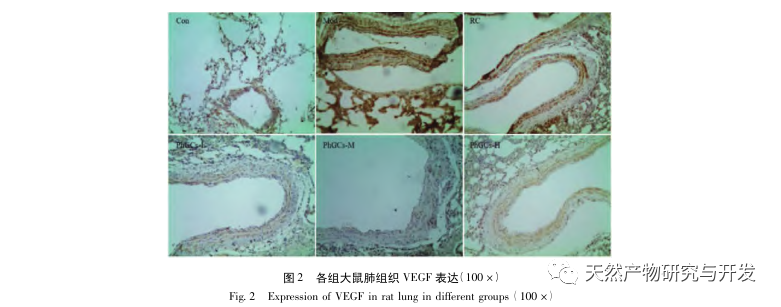
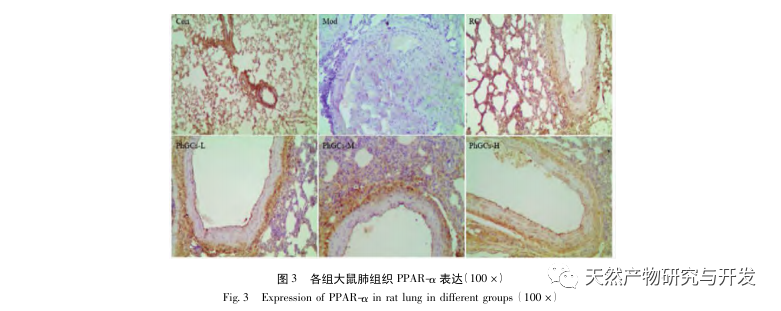
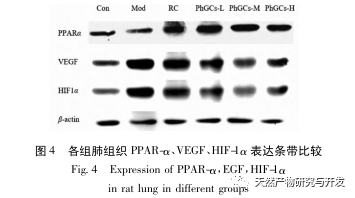
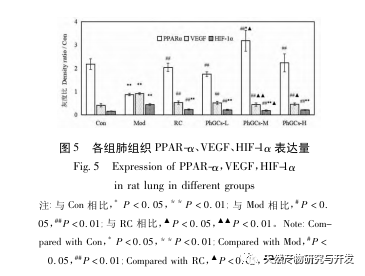
Subsequently, we focused on observing the effects of PhGCs on HIF-1 α, VEGF, and PPAR – α in the hypoxia induced pathway. The expression of mRNA and protein in the lung tissues of rats in each group was detected using immunohistochemistry, fluorescence quantitative PCR, and Western blot methods. Immunohistochemical results showed that the expression level of VEGF in the pulmonary arteries of Mod rats was higher than that of Con, while the expression level of PPAR – α was lower than that of Con. The expression levels of VEGF in the pulmonary arteries of PhGCs low, medium, PhGCs-H, and RC groups were lower than those of Mod, while the expression level of PPAR – α increased. This indicates that PhGCs and Rhodiola rosea can inhibit the expression of VEGF induced by hypoxia, promote PPAR – α expression, and alleviate pulmonary vascular proliferation and myogenesis, thus playing a therapeutic role in CMS. Through experimental measurement of the expression of HIF-1 α protein and mRNA in lung tissue, it was found that the expression of HIF-1 α protein in the lung tissue of CMS model rats significantly increased, while the expression of mRNA and Con showed no difference. This is consistent with the relevant research results showing that hypoxia mainly regulates the expression of HIF-1 α protein. PhGCs can significantly reduce the expression levels of HIF-1 α protein and mRNA, indicating that they may have an impact on the transcription and translation of HIF-1 α. However, Rhodiola rosea had no significant effect on the expression of HIF-1 α mRNA, indicating that it mainly affects protein expression like hypoxia.
Overall, among the three doses of PhGCs, the PhGCs-M group showed the best effect on CMS, while the PhGCs-H treatment did not show significant enhancement, which may be related to the decrease in intestinal absorption rate of PhGCs-H. Compared with Rhodiola rosea, PhGCs-M has a better therapeutic effect on HIF-1 α mRNA and VEGF mRNA indicators. Coupled with resource advantages, PhGCs have certain development prospects.
