Exploring the mechanism of action of emodin in treating sepsis related acute kidney injury based on data mining and experimental verification
Sepsis is a life-threatening organ dysfunction caused by an imbalance in the host’s response to infection. The kidneys are one of the most commonly affected organs in sepsis, and sepsis associated acute kidney injury (SA-AKI) often becomes the direct cause of death in sepsis patients, with a high mortality rate, posing unprecedented challenges for clinical doctors. AKI persists and increases the potential risk of chronic kidney disease (CKD). Surviving patients often inevitably progress to end-stage renal disease (ESRD), posing a serious threat to their quality of life and safety.
Unfortunately, the current ability to prevent and treat SA-AKI is very limited. Active liquid based therapy may not have reliable evidence-based medicine, and may even be harmful. The use of vasoactive drugs to maintain blood pressure requires a balance between large circulation and microcirculation, and there is no consensus on how much blood pressure target should be maintained to help prevent the occurrence of AKI. If renal prevention fails, renal replacement therapy (RRT) must be used for treatment, but the optimal timing and method of RRT intervention are not clear. If SA-AKI patients survive, although most patients’ kidney function will recover, little is known about the mechanisms of kidney repair or failure of kidney function repair, and the lifelong risk of progression to CKD and ESRD is higher. So far, there is no definite and reliable drug for treating AKI. The conventional clinical treatment measures are mainly kidney replacement and symptomatic comprehensive treatment. The repair of kidney function depends on the reliable support of the kidney itself in the body as a whole. Therefore, exploring drugs or measures that can effectively prevent or treat SA-AKI in an early and timely manner, avoid the occurrence of chronic kidney disease or promote kidney repair, has important clinical value for reducing the mortality rate of sepsis patients and improving the quality of life of surviving patients.
Emodin is a natural compound extracted from Chinese herbs such as rhubarb and Polygonum cuspidatum, which has various pharmacological effects such as anti-inflammatory, antiviral, and anti-tumor. Previous studies have confirmed that emodin has a protective effect on sepsis patients; Recent studies have shown that emodin has a protective effect against damage caused by oxidative stress, inflammation, and cell apoptosis. However, it is still unclear whether emodin can improve the prognosis of SA-AKI patients. This study will explore the key genes involved in the pathogenesis of SA-AKI through life science related databases, and analyze the inflammatory signaling pathways involved in these key genes; Furthermore, animal experiments were conducted to investigate whether emodin affects the inflammatory pathway of SA-AKI and its protective effect and mechanism on SA-AKI rats, providing new ideas for its prevention and treatment.
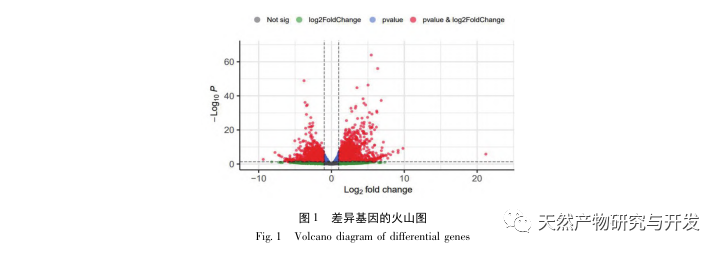
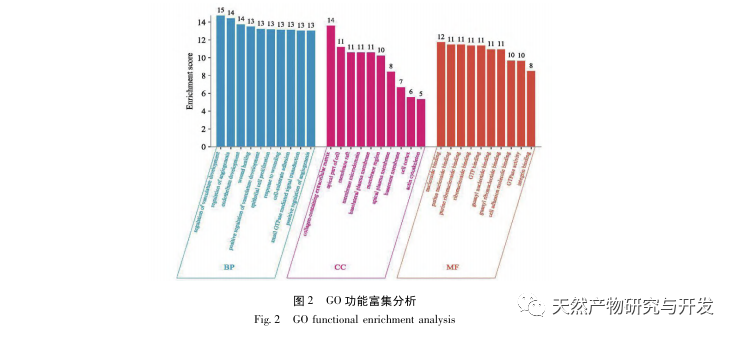
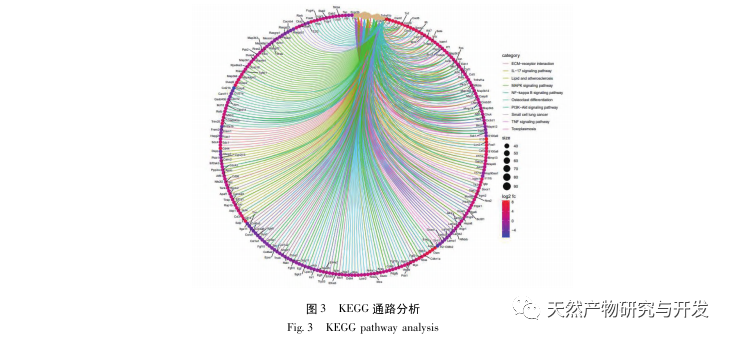


Research has shown that the pathogenesis of SA-AKI is multifactorial and complex, involving the interaction between inflammation, microcirculatory dysfunction, and metabolic reprogramming. Its pathophysiology involves damage and dysfunction of multiple cell types. In the process of sepsis, bacteria release endotoxins or endotoxin like substances, which activate inflammatory cells such as neutrophils, monocytes, and endothelial cells in the body, releasing a large amount of endogenous inflammatory mediators into the bloodstream. On the one hand, this causes damage to multiple organs including the kidneys, and on the other hand, it activates more inflammatory cells to participate in the disease, forming a malignant immune network response. In the SD rat model of lipopolysaccharide (LPS) – induced SA-AKI, nerolidol alleviates SA-AKI by inhibiting the NF – κ B and Toll like receptor 4 (TLR4) signaling pathways. The TLR4/NF – κ B pathway has been confirmed to be involved in the process of renal inflammation response, and inhibiting TLR4/NF – κ B mediated inflammatory response has a protective effect on LPS induced AKI. It can be seen that inflammatory response is an important mechanism in the pathogenesis of SA-AKI, and inhibiting the inflammatory response pathway is an important treatment option for sepsis, providing new ideas for the clinical treatment of SA-AKI patients.
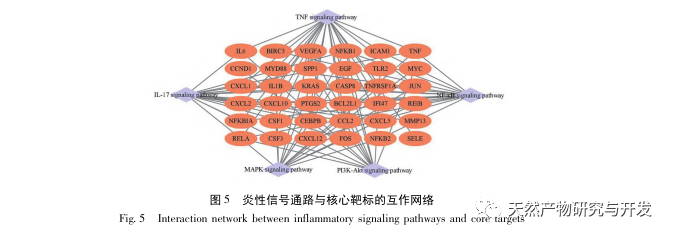
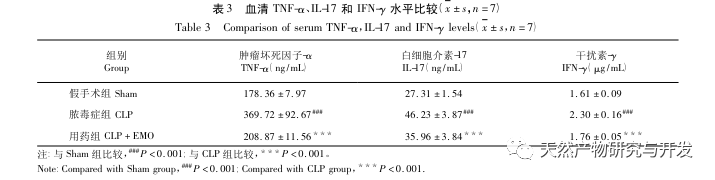
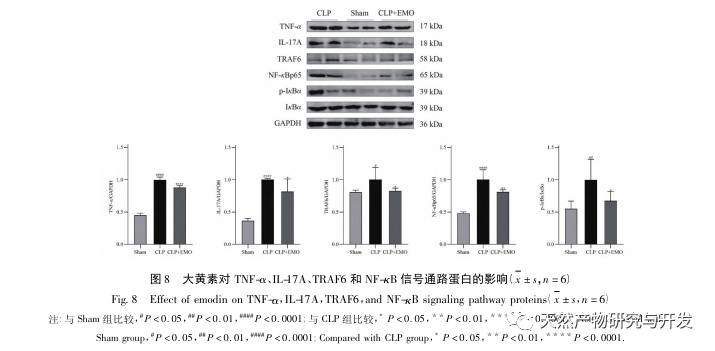
This study utilized a biological database to analyze 2801 key targets involved in the pathogenesis of SA-AKI. KEGG analysis of these targets revealed a total of 5 key inflammatory response signaling pathways, among which the TNF signaling pathway and IL-17 signaling pathway ranked high, and the NF – κ B signaling pathway served as a hub for both pathways to participate in the pathogenesis of SA-AKI. In the TNF signaling pathway, TNF – α is not only the key upstream activator of NF – κ B signaling, but also serves as a downstream responsive molecule of the NF – κ B signaling pathway, suggesting that it promotes the NF – κ B signaling pathway through positive feedback, and the two complement each other. In the IL-17 signaling pathway, IL-17A is the main initiating factor in the IL-17 family, and Th17 cells are its main secreting cells. In the CLP induced model, it was found that IL-17A is highly expressed in the abdominal cavity and plays a key role in the inflammatory response after severe sepsis. Neutralizing IL-17A in the abdominal cavity can reduce the production of pro-inflammatory cytokines. Meanwhile, studies have shown that Toll like receptor 9 (TLR9) in dendritic cells (DCs) may play a key role in the development of SA-AKI by mediating IL-17A production by gamma delta T cells; Other studies have shown that knocking out IL-17A can prevent SA-AKI. The above indicates that TNF – α and IL-17A are pro-inflammatory factors in sepsis, and the TNF signaling pathway and IL-17 signaling pathway are widely involved in the occurrence and development of sepsis, which is consistent with the data analysis results.
There have been many studies on the treatment of sepsis with emodin, mainly focusing on brain, blood, heart, intestinal, and lung tissues. In sepsis associated encephalopathy (SAE), emodin can improve cognitive impairment and pathological damage, and inhibit CLP induced inflammation in mice by upregulating BDNF/TrkB signaling. In the blood system, emodin downregulates P-selectin, improves platelet count and aggregation ability in the late stage of sepsis, and enhances endogenous coagulation factor activity and fibrinogen function, exerting anti-inflammatory effects. In septic cardiomyopathy, it has been found that emodin can reverse cardiac dysfunction and improve myocardial condition in septic rats, which may be related to its inhibition of inflammasome activation. In sepsis induced intestinal injury, emodin can improve intestinal mucosal damage by reducing levels of inflammatory factors and oxidative stress markers, and its mechanism of action may be related to the VDR/Nrf2/HO-1 pathway; And by increasing the expression level of tight junction (TJ) protein, it protects the integrity of the intestinal barrier and inhibits intestinal barrier permeability. In addition to improving the inflammatory response and barrier function of the intestine, emodin can also prevent the displacement of Escherichia coli, prevent the spread and transfer of bacteria, and reduce the secondary harm caused by bacteria. In sepsis related acute lung injury, emodin can inhibit the NF – κ B and high mobility group box 1 (HMGB1) pathways, thereby reducing pulmonary oxidative stress and inflammatory response. Another study on the lungs is based on the autophagy pathway, and the intervention of emodin can effectively prevent the progression of acute lung injury. Moreover, other studies have shown that emodin can effectively alleviate pulmonary tissue edema in sepsis induced acute lung injury by regulating aquaporin (AQP), TJ, inflammatory factors, and lung cell apoptosis. The study of emodin on SA-AKI has not been reported yet. Through this study, it was found that CLP model rats treated with emodin showed decreased expression of TNF – α, IL-17, and IFN – γ in ELISA results, which is consistent with the inhibitory effect of emodin on sepsis inflammation.
The same effect of emodin on the Th17/Treg inflammatory axis has also been elucidated. In the study of acute pancreatitis, it was found that emodin inhibits the immune response in severe acute pancreatitis by regulating the ratio of IFN – γ/IL-17, thereby alleviating intestinal barrier dysfunction. And this study found that in SA-AKI, emodin can also reduce the expression of IL-17A and improve its inflammatory response.
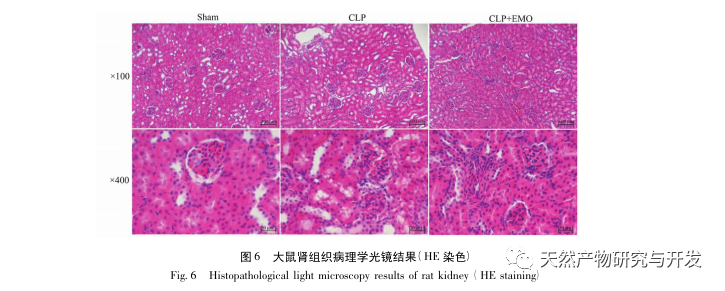
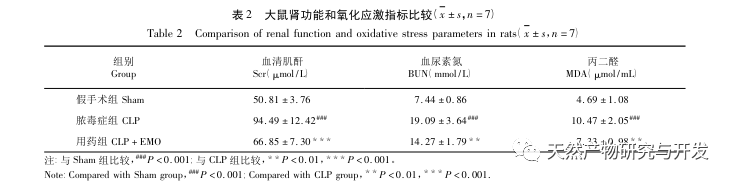
In summary, this study identified 2801 key genes involved in the pathogenesis of emodin through data mining. GO biological process analysis suggests that the biological functions of these targets mainly involve membrane signal transduction, vascular regulation, and wound healing. KEGG pathway enrichment analysis showed that TNF, IL-17, PI3K Akt, NF – κ B, and MAPK signaling pathways were enriched in inflammation related signaling pathways. Experimental verification showed that the treatment with emodin improved renal function in SA-AKI, and the levels of oxidative stress (MDA) and inflammatory cytokines (TNF – α, IL-17, and IFN – γ) decreased. The protein expression of IL-17A, TNF – α, TRAF6, NF – κ Bp65, and the phosphorylation level of I κ B α were significantly reduced compared with the CLP group, which is consistent with the data analysis results. It is suggested that emodin can improve SA-AKI in rats, which may be related to the IL-17/NF – κ B and TNF/NF – κ B signaling pathways.
