Exploring the mechanism of Morinda officinalis against atherosclerosis based on network pharmacology and experimental verification
Atherosclerosis (AS) is a common chronic cardiovascular disease characterized by the formation of atherosclerotic plaque or fibrous plaque in the intima of large and middle arteries. The infiltration of lipids into the arterial intima has become the main pathogenesis of atherosclerosis. Macrophages gathered in the intima and media engulf a large amount of oxidized low density lipoprotein (ox LDL) through scavenger receptors on the cell membrane to form foam cells, which drive the cells to release a large number of proinflammatory factors and matrix metalloproteinases in the intercellular space, promote the accumulation of plaque in the arterial wall to increase, then turn into unstable plaque, and finally progress to myocardial infarction. However, effectively delaying the progress of AS has always been one of the challenges that troubles the academic community. In recent years, the popular emerging medicinal and edible product, Morinda officinalis, has been proven to have good effects in regulating blood lipids and improving atherosclerosis.
Morinda citrifolia, also known as Noni, is a plant of the Euphorbia genus in the Rubiaceae family. It has pharmacological effects such as anti-inflammatory, antioxidant, and lipid-lowering. According to traditional Chinese medicine theory, Morinda officinalis has a sour and sweet taste, a balanced medicinal property, and has the effects of nourishing kidney essence, balancing yin and yang, and delaying aging. It can effectively prevent cardiovascular and cerebrovascular diseases. However, the specific mechanism of Hai Ba Ji in treating AS is still unclear. Given the complex chemical composition of Morinda officinalis, which is mainly composed of anthraquinones, flavonoids, phenylpropanoids, phenolic acids, and other components, in line with the multi-component and multi-target characteristics of traditional Chinese medicine, network pharmacology can be used to clearly elucidate the molecular mechanism of the pharmacological effects of Morinda officinalis. In recent years, network pharmacology has become an emerging discipline that integrates computer science, bioinformatics, and data mining. It constructs a “drug active ingredient disease target disease” network and explains its biological functions, enabling people to have a clearer understanding of the mechanisms and mechanisms of drug therapy for diseases. This study is based on the diverse active ingredients and rich targets of Morinda officinalis, and intends to use network pharmacology methods to predict and analyze the targets, biological processes, and signaling pathways of Morinda officinalis in the treatment of AS. At the same time, in vitro experiments will be used for verification, in order to provide pharmacological basis for drug development and treatment of AS.
 atherosclerosis
atherosclerosis

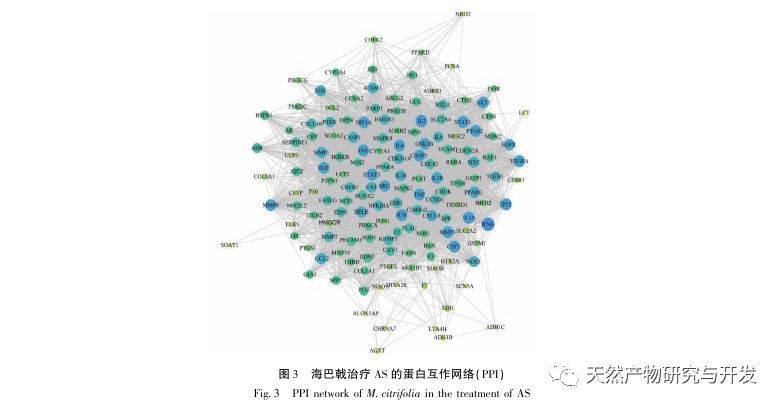
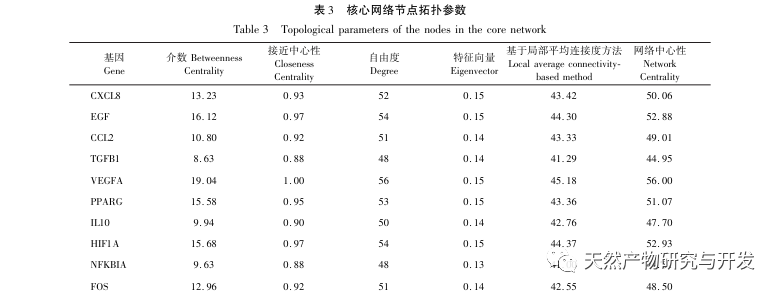
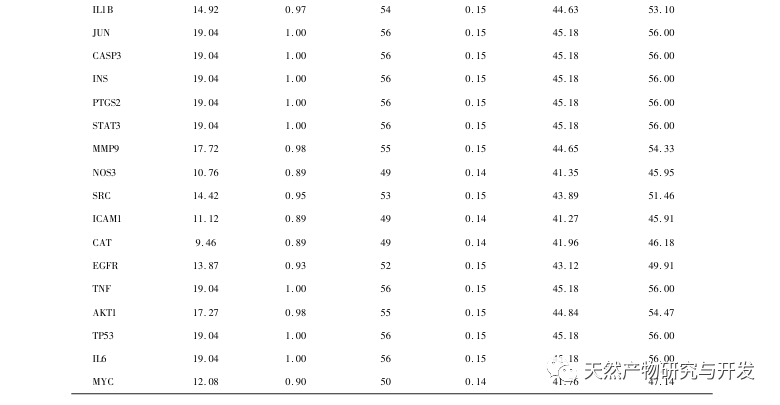
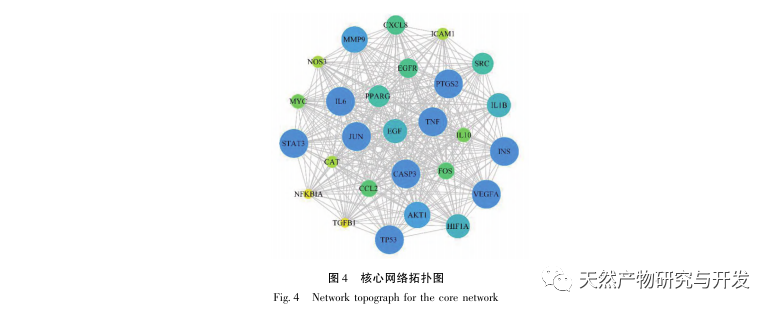
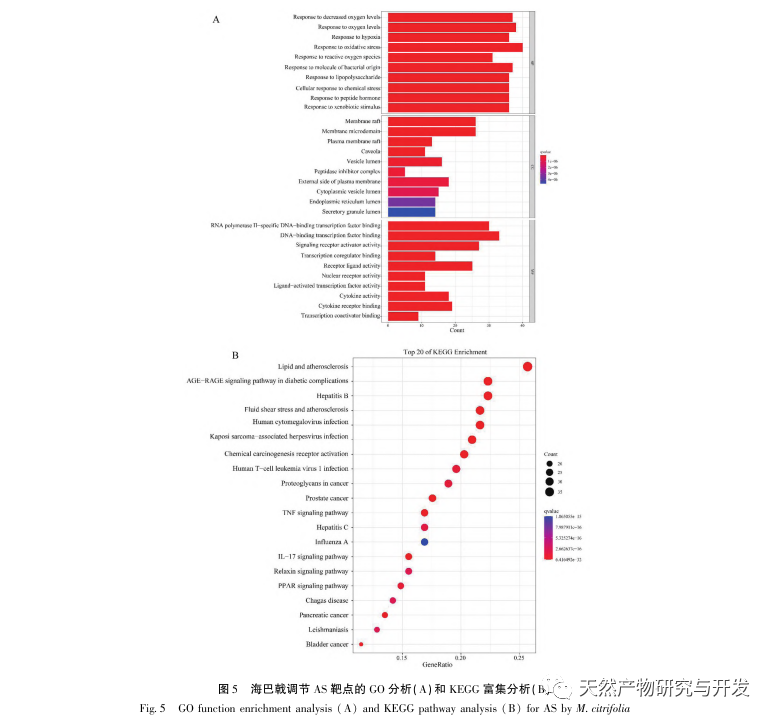
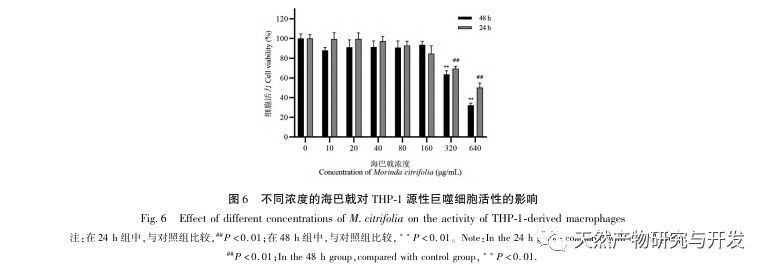
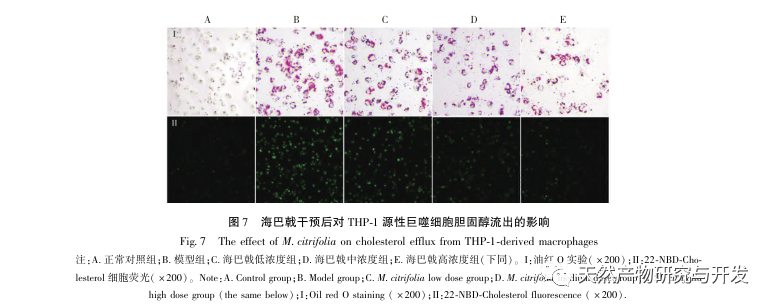
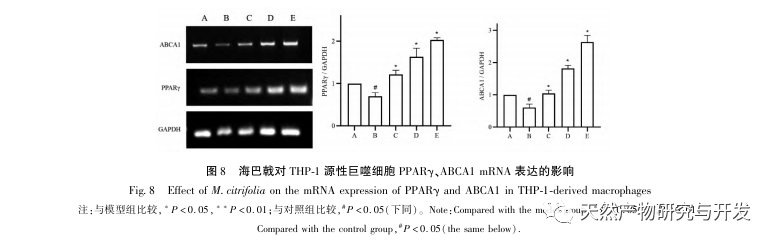
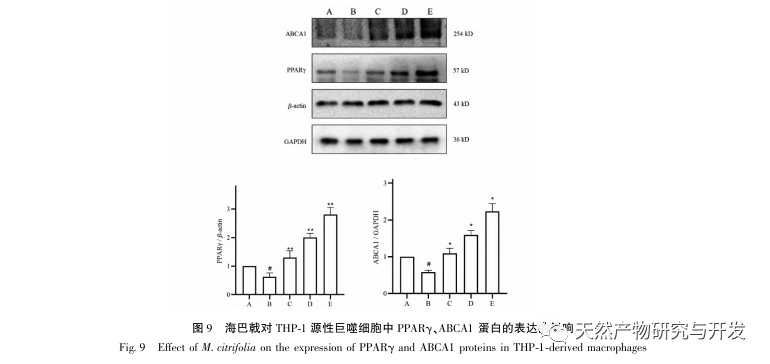
AS is a common cardiovascular disease, and its rapid progression can directly lead to malignant cardiovascular events in patients, such as myocardial infarction. Modern medicine believes that the lipid theory is still the main mechanism of AS occurrence. Statins, PCSK9 inhibitors, and other lipid-lowering drugs are commonly used in clinical practice to slow down the progression of atherosclerosis. However, due to the adverse reactions such as muscle soreness and liver function damage that still occur at therapeutic doses, many patients cannot tolerate these drugs. Therefore, the search for new, safer, and less toxic drugs to treat AS has positive significance. As an emerging medicinal and edible product, Morinda officinalis has a wide range of active ingredients and pharmacological effects, which is in line with the typical characteristics of traditional Chinese medicine’s multi-target and multi pathway treatment of diseases. This study used network pharmacology to predict the effective ingredients and targets of Morinda officinalis in treating AS, and constructed a THP-1 derived macrophage model to verify that Morinda officinalis promotes intracellular cholesterol efflux through the PPAR γ signaling pathway.
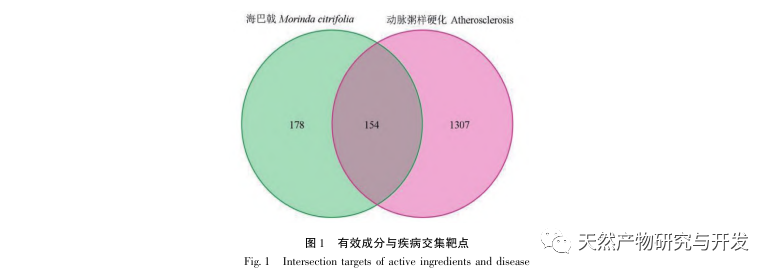
Through screening various databases, it was found that 59 compounds, including flavonoids, phenylpropanoids, and phenolic acids, mainly play a therapeutic role in treating AS. In recent years, multiple research reports have shown that flavonoids play a key role in the treatment of SA and are the main active sites of plant medicine. Sahib et al. found that the contents of catechins, quercetin, and kaempferol in the fruit of Morinda officinalis were 53.68mg/g, 7.4mg/g, and 6.4mg/g, respectively, ranking among the top three known active flavonoids. These three components play a significant role in regulating blood lipid abnormalities and delaying atherosclerosis. Research has found that the main component of Yixin Tongmai granules, catechins, can directly activate the PPAR γ pathway and improve AS through molecular docking and in vitro and in vivo experiments. Quercetin is widely present in various plants, with a significant proportion in Morinda officinalis. Jia et al. and Li et al. reported that quercetin can significantly reduce the plaque content in the thoracic aorta of Apoe -/- mouse AS model induced by high-fat diet. The mechanism is related to the increased expression of ATP binding cassette transporter A1 (ABCA1). Research has confirmed that kaempferol has strong anti-inflammatory activity and can delay the progression of atherosclerosis by activating the PI3K/AKT/Nrf2 pathway.
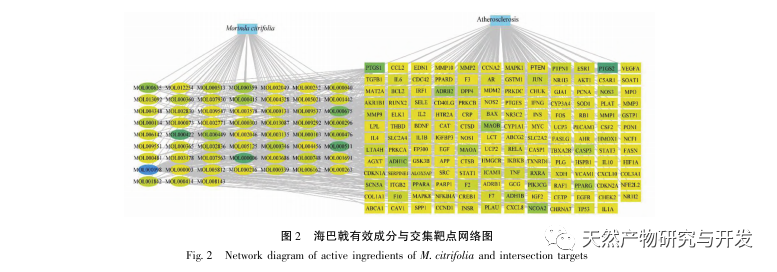
Subsequently, GO enrichment analysis revealed that Morinda officinalis mainly regulates biological functions such as membrane receptors and nuclear receptors in cells; Enrichment analysis of KEGG signaling pathways revealed that the treatment of AS with Morinda officinalis is associated with lipid metabolism related signaling pathways, with the PPAR signaling pathway being one of them. Research has shown that lipid metabolism related signaling pathways, especially the PPAR signaling pathway, are closely related to lipid transporters on the cell membrane and receptors in the nucleus. As we all know, PPARs are classic nuclear receptors located in the nucleus. After receiving the signal of the second messenger, PPARs increase the expression of cholesterol transporters on the cell membrane by activating downstream transcription factors, thereby reducing the accumulation of cholesterol in cells and preventing macrophages from evolving into foam cells. Therefore, PPAR signaling pathway plays an important role in reducing the formation of foam cells in lipid nuclei and delaying AS. In addition, the PPARG gene is also an important node in the core network of the component target network in this study.
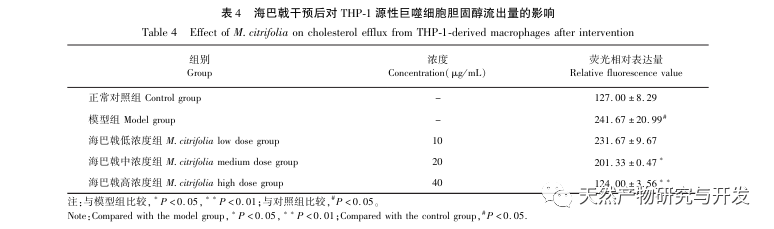
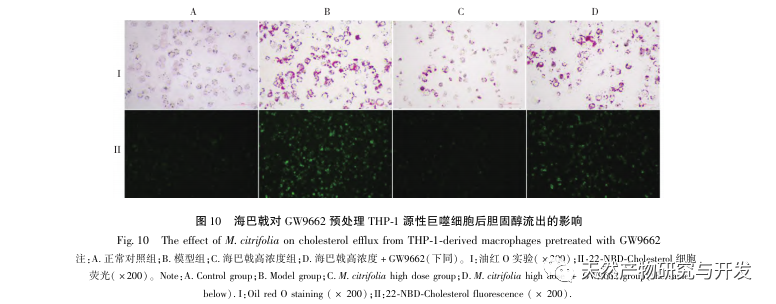
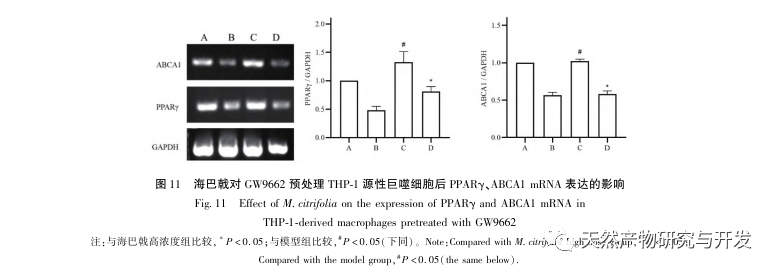
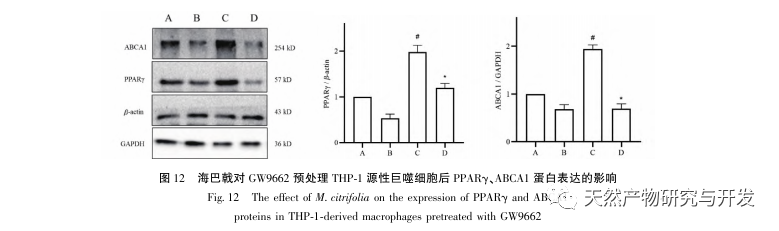
Based on the above network pharmacological analysis, this study constructed THP-1 derived macrophages to simulate foam cells in atherosclerotic plaques in vitro. After Morinda officinalis intervened with THP-1 derived macrophages, it was found that the intracellular cholesterol content was significantly reduced, and the expression of PPAR γ in cells was significantly up-regulated. Inada et al. and Nerurkar et al. respectively confirmed the lipid-lowering effect of Morinda officinalis through animal experiments. In a clinical study, it was found that Morinda officinalis can significantly reduce the serum LDL levels and increase the levels of high-density lipoprotein (HDL) in heavy smoking subjects. Recently, Chong et al. confirmed that Morinda officinalis can significantly reduce lipid plaques in the aorta of a thermally oxidized palm oil diet induced AS rat model, but the specific mechanism has not been elucidated. Earlier, Lee et al. extracted dried powder of Morinda officinalis fruit with 70% methanol and found that it could excite PPAR γ receptors in C2C12 skeletal muscle cells. This experimental report is consistent with our results. This study found for the first time that Morinda officinalis can promote cholesterol efflux from THP-1 derived macrophages, and combined with network pharmacology, predicted its reverse cholesterol transport (RCT) effect by activating the PPAR γ signaling pathway. One of the important mechanisms by which RCT regulates lipid abnormalities and improves AS in vivo is the transfer of cholesterol from cells through scavenger receptors on the cell membrane, which then binds to apolipoprotein A, apoE, and other proteins in the plasma and is transported to the liver for metabolism. It is then converted into bile acids and excreted from the body, thereby improving lipid metabolism abnormalities. ABCA1, as an important scavenger receptor on the macrophage membrane, is finely regulated by the upstream nuclear transcription factor PPAR γ and is responsible for transporting intracellular cholesterol to the extracellular space through the RCT process. In this study, the expression level of ABCA1 was upregulated with the activation of PPAR γ. When PPAR γ inhibitor GW9662 was used, the upregulation trend of ABCA1 receptor expression in THP-1 derived macrophages was inhibited, and there was no significant decrease in intracellular cholesterol content, indicating that ABCA1 mediated by PPAR γ is the main carrier of intracellular cholesterol transport. Similarly, quercetin, baicalin, and dihydromyricetin can all promote RCT and exert anti AS effects by activating the PPAR γ/ABCA1 signaling pathway. Therefore, Morinda officinalis can promote cholesterol efflux from THP-1 derived macrophages by activating the PPAR γ signaling pathway.
In summary, Morinda officinalis has a sweet and sour taste, with a balanced medicinal properties. It can nourish kidney essence, balance yin and yang, and prevent cardiovascular and cerebrovascular diseases. This study used network pharmacology combined with in vitro experiments to reveal the mechanism of Morinda officinalis in treating AS, and for the first time demonstrated that Morinda officinalis can promote cholesterol efflux from THP-1 derived macrophages and promote RCT by activating the PPAR γ signaling pathway. Therefore, this study provides a new pharmacological mechanism for the effective prevention and treatment of AS by Morinda officinalis. However, there are still some limitations to this study: (1) As the TCMSP database did not include the plant medicine Morinda officinalis, the CAMUP database and SwissADME database were used to assist in screening its active ingredients. Although there have been studies using multiple databases to jointly screen traditional Chinese medicine ingredients, the screening criteria for active ingredients in different databases are different, so further verification is needed in terms of screening reliability; (2) This study screened 5 disease-related databases, and the results showed that there were significant differences in the number of AS targets in each database, which may be related to differences in the disease types included in each database; (3) Based on the results of in vitro experiments, our research group will further construct an AS animal model to provide a more complete experimental basis for the promotion of RCT and delay of AS by Morinda officinalis from the perspective of in vivo experiments.
